
US’ Great Point Partners funds the private-equity deal
Pharma supply chain participants are coordinating their response

The Sharing Conference returns to Baltimore in 2017


The pharma industry has a growing number of dependencies on reliable master data

Global efforts to collect clinical data in a more standardized form will lead to more research innovation

“Cold chain” now means cryogenic and ambient shipping, as well as the traditional 2–8° range

Commission issues interim report with numerous calls to action, while manufacturers and distributors contend with multiple DEA penalties




PDUFA reauthorization creates new pathways; GAO report notes FDA’s 99% acceptance rate for expanded access

Global supply chain issues in distribution bring senior execs together

Sales Compensation Solutions, a new book from ZS Associates, outlines problems and solutions

A direct-distribution model could save expense for payers and patients while providing better service

A better way to reverse the cryopreservation common in cellular therapies

A 60% increase in pallet storage capability

Master data on prescribers is combined with tracking expenditures, for transparency reporting

An estimated 64,070 deaths recorded; meanwhile, private equity flows into addiction centers
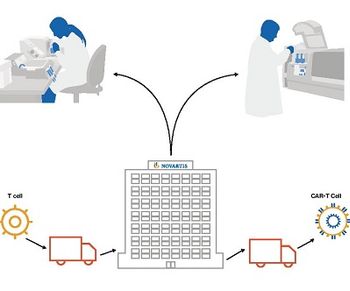
First gene therapy product in US; approval expedited by evidence of high cure rates

Investors hope for a repeat of Gilead’s Sovaldi success

Company is targeting a treatment for glioblastoma; Phase I to begin next year
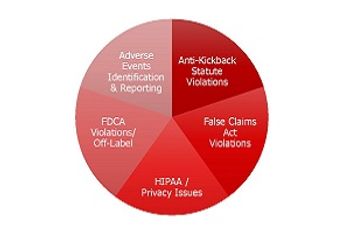
Pharma needs better internal policies to manage its increasing patient interaction

In the midst of the opioid crisis, drug distributors need to address DEA oversight
Observers are critical of the $465-million penalty as too lenient

Leading CRO looks to better trial recruitment, outcomes research

Is the accelerated-approval process providing a net benefit to patients?

Specialized conditioning technology simplifies the shipment prep work

CEIV Pharma certification; expanded temperature regimes and better calibration capabilities