
FDA’s recommended guidance is for a system that ‘does not exist, cannot be built by 2023, and poses unacceptable security and compliance risks,’ says alliance

FDA’s recommended guidance is for a system that ‘does not exist, cannot be built by 2023, and poses unacceptable security and compliance risks,’ says alliance

“Aggregation” and “inference” are specifically allowed, under draft guidance

Pair of life-sciences data powerhouses are immersed in dispute over their respective data services for pharma sales and marketing

Nationally and internationally, the logistics service providers for life sciences see a new vista


Steady progress is occurring in the global effort to track pharmaceutical distribution, even as new technical and business opportunities appear

The goal of producing meaningful data is driving industry progress

Even as the science behind the new therapies is getting established, payers are preparing for their extraordinary cost

A 2017 postponement makes 2018 the key year for serializing US products; authentication makes a comeback, and cargo security goes digital

Patient support (hub) services rise in lockstep with specialty pharmaceutical launches

High prices and questionable marketing activities muddy an otherwise bright picture

Vendors and service providers scramble to serve a growing market

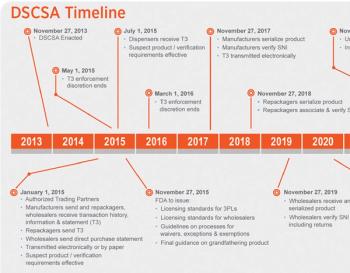
Pharma spends heavily to secure its supply chain, while politicians bring out the re-importation topic

Connectivity with patients drives pharma growth


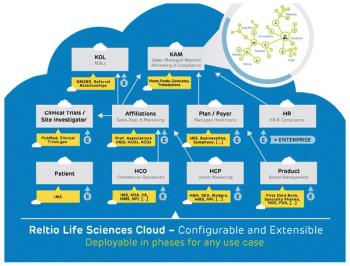
Dramatic changes are seeping into how pharma companies manage diverse datasets

The rise in product volume is more than matched by the rise of regulatory constraints

Looking at the essential value of well-managed supply chains


While FDA tries to referee the naming conventions for biosimilars, different factions of the healthcare system weigh in with conflicting priorities



Packaging-line engineers are going into overdrive as industry gets ready for the 2017 unit-serialization mandate


Contract development and manufacturing organizations (CDMOs) want to be more reliable business partners to pharma

Technology and regulations drive shippers toward more data collection and reporting


New IT tools are propelling a more focused approach to product launch

Published: July 16th 2012 | Updated:

Published: August 27th 2012 | Updated:

Published: August 29th 2012 | Updated:

Published: October 29th 2012 | Updated:

Published: December 27th 2012 | Updated:

Published: December 30th 2012 | Updated: