
Johnson & Johnson’s TAR-200 has a novel targeted releasing system that allows for a controlled release of gemcitabine in patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer.

Johnson & Johnson’s TAR-200 has a novel targeted releasing system that allows for a controlled release of gemcitabine in patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer.
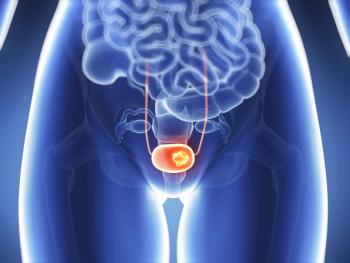
Opdivo (nivolumab) plus cisplatin-based chemotherapy shows improved survival benefits in the first-line treatment of adults with unresectable or metastatic urothelial carcinoma.
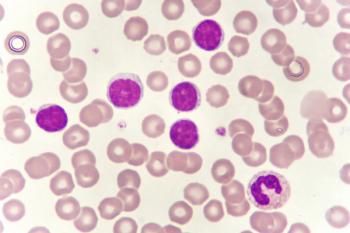
Jaypirca (pirtobrutinib) is indicated for adults with chronic lymphocytic leukemia or small lymphocytic lymphoma who received at least two prior lines of treatment with a Bruton tyrosine kinase inhibitor and a BCL2 inhibitor.
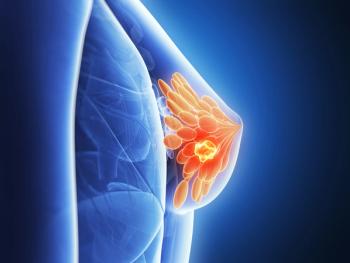
Ongoing clinical trials are evaluating zotatifin (eFT226) plus Faslodex (fulvestrant) and Verzenio (abemaciclib) for the treatment of ER–positive, HER2-negative advanced or metastatic breast cancer.
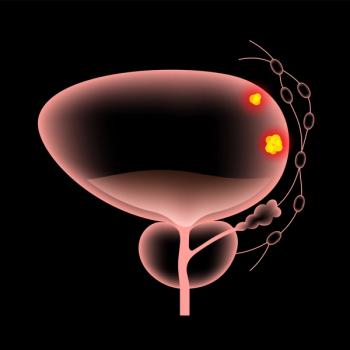
In clinical trials, Keytruda plus Padcev lowered the risk of disease progression or death by 55% compared with chemotherapy in adult patients with locally advanced or metastatic urothelial carcinoma.

Sphingosine-1-phosphate receptor modulator is designed to improve leukemia-free survival by increasing graft-versus-leukemia response.
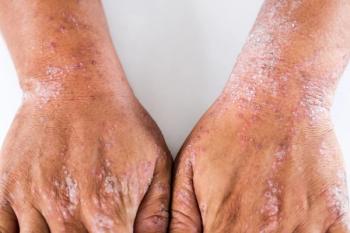
In clinical trials, roflumilast cream demonstrated rapid and sustained improvement in the signs and symptoms of atopic dermatitis in patients 6 years of age and older.

The companies will partner up to design and implement solutions that support each other's business efforts.

In clinical trials, KarXT (xanomeline-trospium) demonstrated a combination of strong tolerability and clinically meaningful symptom reduction in adult patients with schizophrenia.

CDC finds administration rates for influenza antiviral medication was lower than during pre–COVID-19 pandemic flu seasons.

The approval of Ogsiveo represents an important therapeutic advance for patients with progressing desmoid tumors requiring systemic treatment.

The designation—which ranks the fastest-growing companies in North America—is powered by AeroSafe’s 477% growth rate.
AbbVie and Genmab will share commercial responsibilities in the United States and Japan for epcoritamab-bysp (Epkinly) for the treatment of adults with follicular lymphoma that is relapsed or refractory following treatment with two or more therapies.

Alladapt Immunotherapeutics said the FDA Fast Track designation highlights the importance of developing a treatment for multiple food allergens.

IATA numbers point to continuing recovery.

Purchase grows the company’s CRYOPDP domestic footprint while strengthening working relationship between both parties.

The FDA also approved a companion diagnostic test to detect the PIK3CA, AKT1, and PTEN alterations as part of the approval for AstraZeneca’s capivasertib (Truqap) plus fulvestrant (Faslodex) for HR-positive, HER2-negative locally advanced or metastatic breast cancer.
Enzalutamide (Xtandi) gets FDA approval to treat nonmetastatic castration-sensitive prostate cancer with biochemical recurrence at high risk for metastasis.
Pembrolizumab (Keytruda) plus fluoropyrimidine- and platinum-containing chemotherapy for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction cancer approved by FDA after being found to lower the risk of metastasis or death.
ClearNetwork will be available for a plan sponsor’s list of covered generic, branded, and specialty drugs across 65,000 retail pharmacies in the Express Scripts network.
Bristol Myers Squibb’s repotrectinib (Augtyro) approved by FDA to treat locally advanced or metastatic ROS1-positive non-small cell lung cancer.
Patients with kidney failure administered chronic hemodialysis who received DefenCath showed a statistically significant lower incidence of catheter-related bloodstream infections.

New initiative highlights importance of a collaborative approach that allows patients to share their insights and contribute value to studies.

Bayer said it will explore access options for patients with follicular lymphoma currently receiving Aliqopa who have experienced a favorable response to treatment.

A demand-driven methodology for data standardization across multiple streams of acquisition could improve the sharing of real-world data worldwide.
The Flowflex COVID-19 Antigen Home Test is the first over-the-counter rapid antigen test for SARS-CoV-2 to gain FDA 510(k) marketing clearance by the FDA.
Fruquintinib (Fruzaqla) is a significant new therapy for patients who have had limited options to treat metastatic colorectal cancer.

Tirzepatide (Zepbound; Eli Lilly and Company) will hit the US market by the end of 2023 with a list price of $1,059.87.

Rexulti was found to be a well-tolerated treatment for the debilitating symptoms of agitation associated with dementia due to Alzheimer’s disease.

Ayala Pharmaceuticals’ AL102 is a gamma secretase inhibitor being studied to treat patients with desmoid tumors.