When it comes to navigating the complex clinical and commercial landscape for specialty drugs—including hurdles in access to these critical but costly therapies—today’s specialty pharmacies are bringing more skills and services to the table.

When it comes to navigating the complex clinical and commercial landscape for specialty drugs—including hurdles in access to these critical but costly therapies—today’s specialty pharmacies are bringing more skills and services to the table.

By marrying real-world evidence and AI, pharma stakeholders can identify new opportunities to tailor interventions in a way that is most impactful to patients—helping them overcome roadblocks in initiating and maintaining therapy.

How drugmakers can better harness real-world evidence to drive their clinical and commercial objectives, while also providing greater clarity for healthcare providers and improved access to therapy for the patients they serve.

Creating a user-centric approach that harnesses both digital tools and behavioral science is key to getting—and maintaining—patients on therapy, and improving clinical, financial, and, ultimately, brand-success outcomes for pharma manufacturers.

While generics and adherence challenges are keeping drugmakers up at night, breakthroughs in the pipeline coupled with innovative uses of artificial intelligence and machine learning techniques are bringing optimism to the cardiovascular disease space.

Innovation and investment in advancing lifesaving therapies for pediatric patients to the commercial stage remains a daunting task, but rapid advances today in modeling and simulation are reducing the hurdles for drugmakers.
As the range of treatment options for rheumatoid arthritis (RA) continues to grow and biosimilars are poised to enter the arena, the need for expert strategies to improve therapy selection and address the access, affordability, and adherence challenges patients face is coming into sharper focus.

Stakeholders are investing heavily in their ability to curate appropriate data sets and devise advanced data-analytics capabilities to harness real-world evidence (RWE) across the entire pharma lifecycle—from drug discovery and development through product launch and commercialization
While all drug development efforts are fraught with peril, a growing number of biopharma companies—large and small—are staking a claim in the rare disease treatment space, chasing odds that could yield clinical and financial rewards

Pharmaceutical distribution today is anything but monolithic. While companies in this space are grappling with issues ranging from inflation and supply chain disruption to workforce scarcity and DSCSA implementation, there’s still plenty of room for smaller players to stake out part of the terrain to call their own

Proper control of type 1 and 2 diabetes can greatly reduce the personal impact and economic burden of this pervasive public health condition. To that end, stakeholders are exploring many parallel routes to help individuals and society maintain the upper hand, as the toll of diabetes continues to skyrocket
Ongoing challenges—ranging from the growing complexity of today’s advanced therapy options, to inherent shortcomings in the clinical trial process, to ongoing adherence challenges and more—must be reconciled to ensure both clinical and commercial success throughout cancer care

Today’s ideal pharma third-party logistics (3PL) provider brings the right mix of physical assets, internal expertise, data analytics and strategic partnerships to function as a seamless extension of a manufacturer’s internal operations—no matter what disruptions arise

Ongoing strides in data mining, data analytics, modeling and simulation are opening up new and more definitive opportunities to apply data-driven insights to improve everything from upstream drug discovery and clinical trials, to the downstream go-to-market strategy and post-patent lifecycle management

The pressure is on for stakeholders to continue pioneering novel engineering designs, insulation options and advanced monitoring and data analytics tools—to meet the complex demands of today’s temperature-controlled products while keeping costs low

Amid the need for enhanced visibility across the entire pharma ecosystem, strategies focused on ramping up digitalization are critical for manufacturers to boost agility and resilience in the face of predictable and unforeseen threats

A well-oiled, data-driven hub program is critical for stakeholders in supporting patients—and clearing a successful path for specialty therapies

How analytics-driven insights can help drugmakers navigate the leap from trial to market—and add clinical and financial value at every step
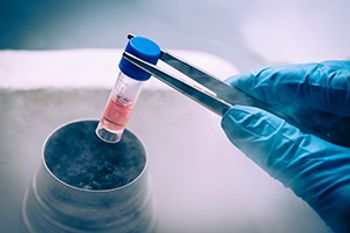
Both COVID-19 vaccine delivery and the growth of cellular/genetic therapies call for lower storage and shipping temperatures

As the definition of ‘targeted’ therapies—especially in oncology—and as a component of companion diagnostics, biomarkers are creating opportunities in drug research and commercialization

Nearly a million US citizens live with HIV, a virus that still lacks a curative therapy

Today's smart, advanced self-injectable devices aim to provide therapeutic value to patient and physicians, and commercial value to the drug makers and payers

Vaccines for adult disease states and for tropical diseases expand the market beyond traditional pediatric applications

A mix of high-tech and high-touch interventions address one of healthcare’s most pressing issues
Even though recombinant-DNA biologics are displacing traditional blood-sourced therapeutics, maintaining stable supplies is still a problem
Good growth is occurring in the vaccine business, despite the hurdles of public perceptions and public-health funding shortfalls
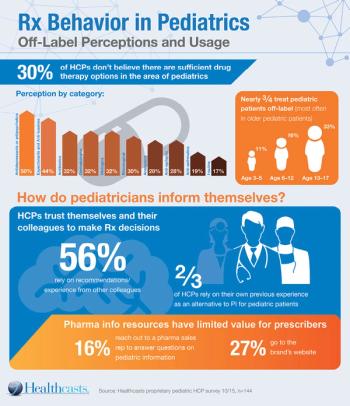
Even with legislative incentives, pharma manufacturers struggle to cost-justify pediatric innovation

With competing products and pricing pressure, the rare disease market is starting to resemble the conventional drug market

While the biopharma industry continues to develop better treatment options, there is a stronger focus on more intensive patient engagement

As new cancer drugs with promising efficacy but brutal costs enter the market, providers, payers and manufacturers are strategizing new payment approaches

Published: June 7th 2024 | Updated:

Published: June 17th 2021 | Updated:

Published: September 23rd 2021 | Updated:

Published: March 19th 2021 | Updated:

Published: August 7th 2023 | Updated:

Published: March 31st 2023 | Updated: