
Technology is enabling more resources to reach the medical affairs teams—in a compliant manner

Technology is enabling more resources to reach the medical affairs teams—in a compliant manner


Managing the physical movement of specialty drugs calls for dedicated services

As the pharma industry moves ahead with traceability initiatives, vendors are building their capabilities

The growing compliance requirements for distribution call for a streamlined approach



The scale of the cardiovascular market pushes payers to demand quality outcomes data

The annual Operation Pangea nets a next crop of crooks; consumer groups raise awareness



The biologics development and approval process for pharmaceutical companies around the world is typically long and complex, which increases the risk of inaccurately forecasting demand
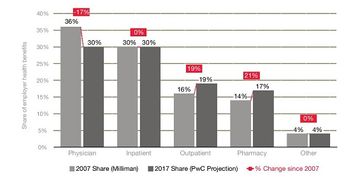
A repeat of the +6.5% cost trend indicates that medical costs are still rising above inflation

Panama Papers point to secret merger between distributors

Reltio Cloud master-data management will glue together IMS data resources

New Kakegawa, Japan, facility will handle storage and packaging

Internationalization of drug wholesaling is a factor in the name change

New physician’s form is supposed to reduce the complexity of obtaining investigational drugs

Branded products are 92% US market in dollar value, says IMS Institute

Prestigious competition for budding scientists takes a biotech vibe

Pilot program in Rwanda has commercial potential

Looming EU IDMP mandates are only part of the potential applications