
The 3PL plans to convert all its trucks to electric by 2027.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Jeb Hunter, Senior Regulatory Consultant, EAS Consulting Group, discusses the on “What to Expect When They’re Inspecting: FDA Inspections on DSCSA Compliance" breakout session at the 2024 HDA Traceability Seminar.
The joint venture aims to disrupt pathological lncRNA interactions with RNA-binding proteins using NextRNA’s platform.

Session uncovers distributors’ experiences with the Waiver, Exception, or Exemption request process, and how these affect the overall supply chain.

The agency provides an update surrounding the Act’s implementation efforts.
The new vials of Zepbound are available through a new self-pay pharmacy channel, priced over 50% lower than other incretin medications for obesity.

Ryan Sevek, the supplier’s CFO, will become president.

Chris Williams expands on the services that a third-party logistics (3PL) partner should offer, while sharing ways to alleviate standard supply chain issues.

A prediction of how this shift toward patient-centric care may look.
The updated COVID vaccine replaces previous bivalent vaccines, focusing on a single strain for more effective immunity.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Meghan Mullooly, Archbow Consulting’s Vice President, outlines the various types of available digital solutions when delivering patient services, while also sharing ways to drive efficiencies.

The unveiling of the microbial fermentation manufacturing plant is headlined by a $131 million financial commitment.

In the final part of his Pharma Commerce video interview, Matt Hawkins, CaryHealth’s Chief Technology Officer, comments on the importance of mitigating security risks, along with ways to take action.
Although it still has to undergo the preclinical and clinical trial process, the DNA vaccine—which could be created in as little as six weeks—does not require the cold chain storage that mRNA jabs often demand.
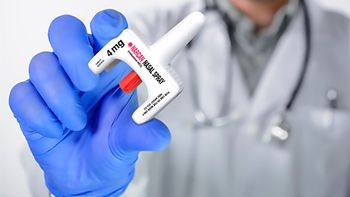
A secret shopper survey study investigates how an FDA approval impacts patient access to a medication used to combat drug opioid overdose.

In this part of his Pharma Commerce video interview, Matt Hawkins, CaryHealth’s Chief Technology Officer, explains the value of AI in the pharmacy environment.

The 86,000 square-foot Køge facility will be fully functional by 2027.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Matt Hawkins, CaryHealth’s Chief Technology Officer, describes how artificial intelligence can not only diagnose patients, but also deliver accurate medical advice.

By 2026, the first 10 drugs for individuals with Part D coverage—whose medications are mainly used to treat cancer, diabetes, and heart disease—will see discounts off of list prices ranging from 38-79%.

In the final part of his Pharma Commerce video interview, Michael Rowe, Two Labs’ Senior Director of DSCSA/Serialization Compliance Services, comments on other ways to limit ‘bad actors’ attempts to sneak counterfeit drugs into the supply chain, aside from the DSCSA.

Partnership adds supply chain efficiencies, while prioritizing patient safety.

The facility will focus on furthering RNA and DNA-based therapies.

In this part of his Pharma Commerce video interview, Michael Rowe, Two Labs’ Senior Director of DSCSA/Serialization Compliance Services, describes the outcome of the joint public meeting held by the Partnership for DSCSA Governance and the FDA.

The partnership creates an integrated solution that also tackles compliance challenges within the sector.

In the final part of his Pharma Commerce video interview, Josh Marsh, Vice President and General Manager of Cardinal Health Sonexus Access and Patient Support, defines the qualifying characteristics of a specialty lite product, and summarizes the main points of his Asembia panel.

The latest news for pharma industry insiders.
The collaboration—which is now active until 2031, features a $25 million payment to Evotec for progress made in neurodegenerative disease research.

What does the legislation mean for patient access leaders, pharma brand managers, and patient service owners? Industry experts weigh in.

In this part of his Pharma Commerce video interview, Josh Marsh, Vice President and General Manager of Cardinal Health Sonexus Access and Patient Support, discusses the impact technology has in relation to patients sticking to their medication plan.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Josh Marsh, Vice President and General Manager of Cardinal Health Sonexus Access and Patient Support, outlines the main challenges surrounding medication adherence.