
The designation—which ranks the fastest-growing companies in North America—is powered by AeroSafe’s 477% growth rate.

The designation—which ranks the fastest-growing companies in North America—is powered by AeroSafe’s 477% growth rate.
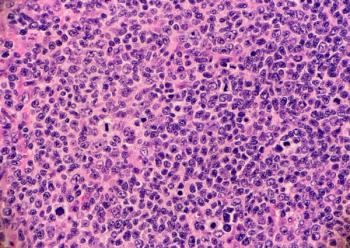
AbbVie and Genmab will share commercial responsibilities in the United States and Japan for epcoritamab-bysp (Epkinly) for the treatment of adults with follicular lymphoma that is relapsed or refractory following treatment with two or more therapies.

Alladapt Immunotherapeutics said the FDA Fast Track designation highlights the importance of developing a treatment for multiple food allergens.

Study aims to find ways industry gets involved in the most influential clinical trials, and how transparent these trials are.

The CDMO’s GMP plant will dedicate 2,500 square feet to the storage of finished products.

IQVIA report explores why these shortages are increasing, and how they can be mitigated.

Medication focuses on the acute treatment of migraines.

Study emphasizes role in bridging research and development and commercial/marketing functions.

IATA numbers point to continuing recovery.

Purchase grows the company’s CRYOPDP domestic footprint while strengthening working relationship between both parties.
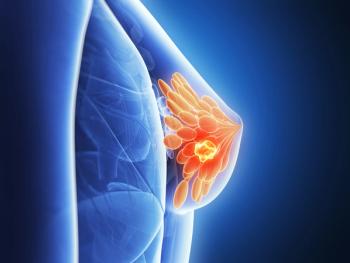
The FDA also approved a companion diagnostic test to detect the PIK3CA, AKT1, and PTEN alterations as part of the approval for AstraZeneca’s capivasertib (Truqap) plus fulvestrant (Faslodex) for HR-positive, HER2-negative locally advanced or metastatic breast cancer.
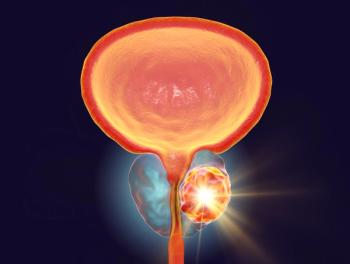
Enzalutamide (Xtandi) gets FDA approval to treat nonmetastatic castration-sensitive prostate cancer with biochemical recurrence at high risk for metastasis.
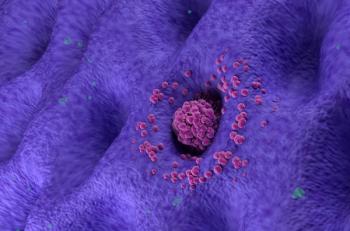
Pembrolizumab (Keytruda) plus fluoropyrimidine- and platinum-containing chemotherapy for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction cancer approved by FDA after being found to lower the risk of metastasis or death.
ClearNetwork will be available for a plan sponsor’s list of covered generic, branded, and specialty drugs across 65,000 retail pharmacies in the Express Scripts network.
Bristol Myers Squibb’s repotrectinib (Augtyro) approved by FDA to treat locally advanced or metastatic ROS1-positive non-small cell lung cancer.
Patients with kidney failure administered chronic hemodialysis who received DefenCath showed a statistically significant lower incidence of catheter-related bloodstream infections.

Findings suggest that participants nationwide who were offered and accessed their online medical record has more than doubled in the past eight years, but racial and ethnic disparities continue.

Parties will provide processing and packaging machinery services.
Study estimates that up to 20% of the costs for clinical research is associated with the manual transfer and verification of data from the electronic medical record to a data capture system.

New initiative highlights importance of a collaborative approach that allows patients to share their insights and contribute value to studies.
Stem Cell Reports takes a look at DTC online advertising claims that these are ‘immune boosters.’

Stephanie L. Woerner, PhD, director and principal research scientist at the MIT Center for Information Systems Research, discusses the outlook for next year among biopharma firms.

Bayer said it will explore access options for patients with follicular lymphoma currently receiving Aliqopa who have experienced a favorable response to treatment.

JMA Journal report describes how businesses in this Asian-Pacific nation are employing various strategies to reduce GHG emissions.

A demand-driven methodology for data standardization across multiple streams of acquisition could improve the sharing of real-world data worldwide.
Exparel was initially approved in 2011 for single-dose infiltration into a surgical site.
The Flowflex COVID-19 Antigen Home Test is the first over-the-counter rapid antigen test for SARS-CoV-2 to gain FDA 510(k) marketing clearance by the FDA.

New E2E workflow orchestration service is expected to simplify the processes of health systems and retail pharmacies while also reducing the risk of recalled items reaching patients.
Fruquintinib (Fruzaqla) is a significant new therapy for patients who have had limited options to treat metastatic colorectal cancer.

Tirzepatide (Zepbound; Eli Lilly and Company) will hit the US market by the end of 2023 with a list price of $1,059.87.