
In the first part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Tom Dorsett, CEO, RazorMetrics, explains the concept of polypharmacy, including how prominent it is in the United States today.
Nicholas Saraceno is Editor of Pharmaceutical Commerce. He can be reached at [email protected].

In the first part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Tom Dorsett, CEO, RazorMetrics, explains the concept of polypharmacy, including how prominent it is in the United States today.

James D. Chambers, PhD, MPharm, MSc, professor of medicine at the Tufts Medical Center Institute for Clinical Research and Health Policy Studies, dives into why biosimilars contribute to a reduction in cost and increase in patient access, while highlighting challenges to adoption.

In the final part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Laura Johnson, senior director of sales, life sciences, Loftware, comments on other concepts that are powering the company’s 2025 Supply Chain Trends Report, and how they could possibly change in 2026.
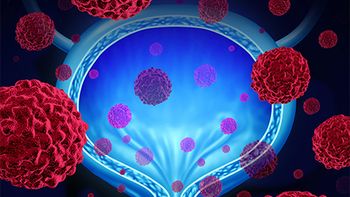
FDA approves an expanded access program that would offer patients an alternative to Merck’s bladder cancer medication.

In the third part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Laura Johnson, senior director of sales, life sciences, Loftware, discusses why the demand for supply chain transparency is continuing to gain momentum, while also explaining the role that track-and-trace initiatives are playing in that effort.

The timing for the potential tax is yet to be determined, as the industry awaits to see the impact it could bring.

A qualitative study examines how Medicaid telehealth reimbursement policies affect staffing and patient-centered care.

In the first part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Laura Johnson, senior director of sales, life sciences, Loftware, comments on why she believes supply chain collaboration is becoming increasingly critical today.
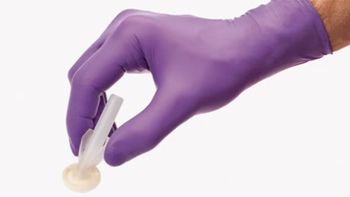
MedTech company issues a risk statement, noting fungal contamination.

In the final part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Jim Shehan, chair of the FDA regulatory practice at Lowenstein Sandler, explains how he anticipates the evolving regulatory environment impacting the future of pharmaceutical development, especially in the context of emerging technologies like AI.

The company’s Mourenx plant in Southwest France aims to become an API network hub, with first supplies expected by next year.

In the final part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Jenna Dale, director of client relations at Cencora, describes why these pharma supply chain stakeholders are vital in understanding meaningful clinical outcomes.

These taxes on goods—with the potential to take effect in weeks or months—will vary by country, but rates could depend on regulations, industry subsidies, and other factors.

In the fourth part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Jenna Dale, director of client relations at Cencora, explains the value of FDA guidance documents for manufacturers, along with the areas of CGT development that would benefit most from standardization.

The facilities are expected to offer pharma shipment support by addressing time and temperature-specific requirements.

Digging a little deeper into the drug class phenomenon reveals some interesting takeaways on a smaller subgroup of users.

In the third part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Jenna Dale, director of client relations at Cencora, dives into specific areas where FDA investment could have the greatest impact in accelerating approval timelines.

Trade & Channel Strategies conference explores the frontline issues related to strategy, distribution, and reimbursement—while digging into creative solutions to solve them.

Trump raises the import tax to 25% on the former—while restoring the latter to 25%—in a move that could potentially take effect on March 4.

Leaders with top third-party logistics providers discuss the evolving landscape for the sector in 2025—and the new strides made in their quest to help transform the pharma supply chain.

In the second part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Jim Shehan, chair of the FDA regulatory practice at Lowenstein Sandler, discusses some of the other specific regulatory updates that drug developers be closely monitoring in the current landscape.
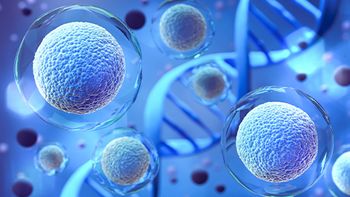
The agreement is centered around improving cell culture efficiency and CGT quality.

In the second part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Jenna Dale, director of client relations at Cencora, explains the challenges associated with meeting the FDA’s 2025 CGT forecasted approval goal.

A cohort study investigates the magnitude of lost coverage among pediatric patients, following the conclusion of the pandemic public health emergency.

In the final part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Alex Guillen, global pharma and life sciences director at Tive, shares how the development of technology will help bolster the pharma logistics industry.

A survey study of US employers aims to determine whether they prioritize financial or nonfinancial criteria when selecting health plans.

In the first part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Jim Shehan, chair of the FDA regulatory practice at Lowenstein Sandler, describes what the approach of pharma companies should be amid the latest/pending tariffs imposed on Canada, Mexico, and China, along with their potential impact on the pharma supply chain.

The company cites a decline in sales and excess inventory as the main contributing factors.

In the first part of her video interview with Pharma Commerce Editor Nicholas Saraceno, Jenna Dale, director of client relations at Cencora, shares how the imposed/pending trade tariffs impact pharma manufacturers.

However, the 10% tariff on imports from China went into effect this morning, with the nation imposing several of it own.