The FDA assigned Autolus Therapeutics' biologics license application for obecabtagene autoleucel with a Prescription Drug User Fee Act date of November 16, 2024.

The FDA assigned Autolus Therapeutics' biologics license application for obecabtagene autoleucel with a Prescription Drug User Fee Act date of November 16, 2024.

The definitive merger agreement was reached in October 2023, with Bristol Myers Squibb acquiring Mirati for $58 per share in cash, for a total equity value of $4.8 billion.

Offerings include treatment plans for a considerable number of illnesses and conditions, company says.

Study describes how a game theoretic model can successfully analyze how adoption of blockchain technology can reveal quality information.

Data from prior Phase II study showed signs of pridopidine slowing disease progression in patients with amyotrophic lateral sclerosis.
NK010, which showed promising anti-tumor activity and safety in preclinical studies, will be evaluated in a Phase I clinical trial for ovarian cancer.
The addressable treatment market for osteopenia is projected to reach more than $30 billion in the United States and $100 billion globally.

Acquisition is expected to strengthen company’s CDMO business, while establishing a footprint in the generics space.
Phase Ib study evaluated satri-cel in patients with advanced gastric/gastroesophageal cancer or pancreatic cancer who had progressed on or were intolerant of prior systemic therapy.
Opdivo (nivolumab) with Yervoy (ipilimumab) showed a 79% drop in the risk of disease progression or death compared to chemotherapy in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer.

Sandoz will buy Cimerli (ranibizumab-eqrn), an interchangeable biosimilar to Lucentis (ranibizumab), for an upfront cash payment of $170 million.
Today's approval amends the accelerated approval granted by the FDA in April 2019 to Balversa (erdafitinib) for patients with metastatic urothelial carcinoma with susceptible FGFR2 or FGFR3 alterations following prior treatment with platinum-containing chemotherapy.
Experts explore ways in which the financial sector refined the healthcare landscape, including its evolutions throughout the years.

In an interview with Pharma Commerce editor Nicholas Saraceno, Bill Roth, General Manager, Managing Partner, Blue Fin Group, discusses the changing role of PBMs and recent price structure overhauls.
Data presented at the 2024 American Society of Clinical Oncology Gastrointestinal Cancers Symposium show first-line Lutathera plus long-acting release octreotide lowered the risk of disease progression or death by 72% in patients with advanced gastroenteropancreatic neuroendocrine tumors.

All healthcare stakeholders must participate to dismantle barriers and ensure access to medicines that save lives.
Application seeks approval for tumor-treating fields therapy combined with either an immune checkpoint inhibitor or docetaxel in patients with metastatic non-small cell lung cancer following progression on or after the use of platinum-based therapy.

In an interview with Pharma Commerce editor Nicholas Saraceno, Bill Roth, General Manager, Managing Partner, Blue Fin Group, discusses changes that will come with the pharma giant's new platform.
Pivmecillinam (Pivya) has a clinical cure rate of 95% in uncomplicated urinary tract infections and is recommended as a first-line option for treatment in many countries.
Data show daily oral dosing of NX-5948 in patients with relapsed or refractory B-cell malignancies experienced a clinical benefit across dose ranges from 50 mg to 200 mg.

The predicted value is largely driven by manufacturers' prioritization of brand protection.
Bloomlife’s MFM-Pro enables healthcare providers to measure maternal and fetal heart rate in the patient's home or clinic.

EdenHelp is expected to provide HCPs and patients with real-time access to Rx drug and medical device information.

Patients with chronic inflammatory demyelinating polyneuropathy administered HyQvia showed a statistically significant difference between relapse rates compared with placebo.

JAMA Health Forum details the DSCSA and stresses the value of protecting the pharmaceutical supply chain from adulterated products.
Researchers from the University of Cambridge collaborated with Pfizer to develop a platform combining automated experiments with artificial intelligence to predict chemical reactions.

Analysis examines how social and healthcare factors play a role.

Initiative seeks to find new therapies to improve the lives of patients with Rett syndrome.
The list price of Casgevy for transfusion-dependent beta thalassemia has yet to be released, however, the cost for the treatment in sickle cell disease is $2.2 million.
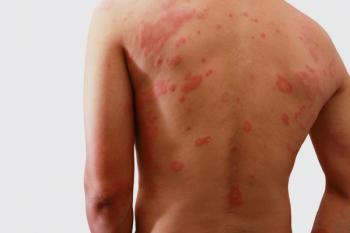
Zoryve (roflumilast) shows efficacy in seborrheic dermatitis and atopic dermatitis in clinical trials.