
The company boosts its healthcare logistics services by agreeing to purchase Frigo-Trans and its sister company BPL.

The company boosts its healthcare logistics services by agreeing to purchase Frigo-Trans and its sister company BPL.

The project is funded by Scottish Enterprise, and aims to boost commercial manufacturing of injectable drug product.

In the second part of her interview with Pharma Commerce Editor Nicholas Saraceno, Meghan Mullooly, Archbow Consulting’s Vice President, offers advice to pharma manufacturers for successfully applying digital solutions to their patient support programs.

The enhancement—which complements the CDMO’s adeno-associated viral vector services—will be utilized at the company’s advanced therapies site in Stevenage, UK.

In the final part of her interview with Pharma Commerce Editor Nicholas Saraceno, Ilisa B.G. Bernstein, President of Bernstein Rx Solutions, LLC, comments on industry’s preparedness for DSCSA compliance and provides an update as to where pharma is in terms of RFID label adoption.

This year’s conference—taking place in Washington, DC— will prioritize the tackling of business, economic, and healthcare policy obstacles when it comes to decision marking and net revenue optimization.

In the second part of her interview with Pharma Commerce Editor Nicholas Saraceno, Ilisa B.G. Bernstein, President of Bernstein Rx Solutions, LLC, discusses the best practices that compliance teams should consider as the end of the stabilization period approaches this November.

The project features several enhancements, including a new 67,000 square-foot warehouse at its HQ.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Ilisa B.G. Bernstein, President of Bernstein Rx Solutions, LLC, highlights the hard-hitting points that she anticipated will be covered during her session at the 2024 HDA Traceability Seminar.

How can industry stakeholders ensure the quality and supply chain security of existing product?

The 8,000 square-foot facility—located within the wholesaler’s Le Vergne distribution center— is expected to help tackle cell and gene therapy logistical challenges.

The company also participated in the coalition’s pilot inspection program, making it the first CDMO to do so.

The 3PL plans to convert all its trucks to electric by 2027.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Jeb Hunter, Senior Regulatory Consultant, EAS Consulting Group, discusses the on “What to Expect When They’re Inspecting: FDA Inspections on DSCSA Compliance" breakout session at the 2024 HDA Traceability Seminar.
The joint venture aims to disrupt pathological lncRNA interactions with RNA-binding proteins using NextRNA’s platform.

Session uncovers distributors’ experiences with the Waiver, Exception, or Exemption request process, and how these affect the overall supply chain.

The agency provides an update surrounding the Act’s implementation efforts.
The new vials of Zepbound are available through a new self-pay pharmacy channel, priced over 50% lower than other incretin medications for obesity.

Ryan Sevek, the supplier’s CFO, will become president.

Chris Williams expands on the services that a third-party logistics (3PL) partner should offer, while sharing ways to alleviate standard supply chain issues.

A prediction of how this shift toward patient-centric care may look.
The updated COVID vaccine replaces previous bivalent vaccines, focusing on a single strain for more effective immunity.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Meghan Mullooly, Archbow Consulting’s Vice President, outlines the various types of available digital solutions when delivering patient services, while also sharing ways to drive efficiencies.

The unveiling of the microbial fermentation manufacturing plant is headlined by a $131 million financial commitment.

In the final part of his Pharma Commerce video interview, Matt Hawkins, CaryHealth’s Chief Technology Officer, comments on the importance of mitigating security risks, along with ways to take action.
Although it still has to undergo the preclinical and clinical trial process, the DNA vaccine—which could be created in as little as six weeks—does not require the cold chain storage that mRNA jabs often demand.
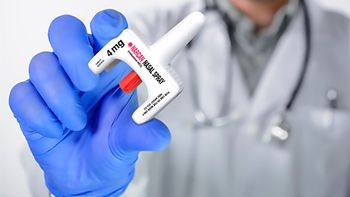
A secret shopper survey study investigates how an FDA approval impacts patient access to a medication used to combat drug opioid overdose.

In this part of his Pharma Commerce video interview, Matt Hawkins, CaryHealth’s Chief Technology Officer, explains the value of AI in the pharmacy environment.

The 86,000 square-foot Køge facility will be fully functional by 2027.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Matt Hawkins, CaryHealth’s Chief Technology Officer, describes how artificial intelligence can not only diagnose patients, but also deliver accurate medical advice.