
Orchestrated Customer Engagement (OCE) breaks down functional silos in commercial operations

Orchestrated Customer Engagement (OCE) breaks down functional silos in commercial operations

Sonoco ThermoSafe's advice on providing value for your product

Latest report tracks growth of specialty sector shows a jump in exclusive distribution
Optel Vision acquires Verify Brand, while rfXcel picks up Frequentz assets

HDA 2017-8 Factbook details rising wholesaler involvement

Acquisition brings retail pharmacy and PBM together with a health insurer

IQVIA’s eighth annual survey shows 77% of respondents go beyond compliance reporting

Private equity firm acquires UBC

The Netherlands wins out in the competition to move the agency from London

$815-million deal to close in early 2018

TrialCard and Careform get new owners

Companies consolidate under 1st Choice Delivery brand

Traceability hardware vendor spreads its wings
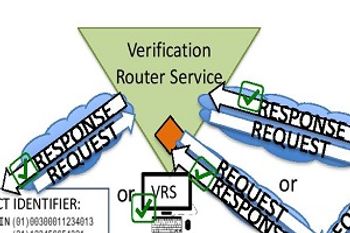
Wholesaler members ready themselves for DSCA-compliant returns processing in 2019


FDA plods along; industry builds out barcode installations, and IT experts pay closer attention to blockchain technology

An inside look at selection and implementation of passive thermal protection


New IT platform modernizes the patient support data-gathering process

A look into the growing compliance risks associated with patient services



Rumors of a $60-billion acquisition shake up healthcare services landscape—and what about Amazon?

Counterfeit opioids and fake medical devices were a focus in the annual take-down of illicit distributors

Accenture survey focuses on healthcare providers’ experiences

IoT takes you from living in the past to shaping the future


Details on transition will be forthcoming
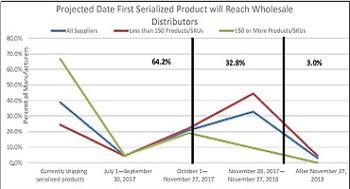
Equipment availability is cited as a cause for delay