
Merger of two giants in their respective fields has global implications

New software solutions and an updated Revenue Cloud for Pharma

Most proposed solutions are already in the works

Acquires a government pricing and contracting data provider

Organizations will collaborate on a proposed GS1 standard

Savsu’s evo DV containers will be available through World Courier’s 140 offices

wo UK companies join forces for pharma cold chain

Another attempt by payers to control their healthcare costs; possible broader implications

‘Shocking’ ease of buying and obtaining delivery of opioids has implications for drug importation

A mix of high-tech and high-touch interventions address one of healthcare’s most pressing issues

Accenture research shows heightened consumer awareness, and boomer/millennial differences

Deal brings Celgene into the CAR-T arena

Acquired company lasted less than a year after Biogen spinoff

Agency plans to issue 'not yet classified' recall notices

The appearance of deep-frozen products will complicate distribution practices

Multi-vendor Team-Up approach should ease systems integration

'Hybrid' service links Marken transportation with its UPS parent

Said to be industry’s first for combined EU, USP and WHO standards

Higher quality service for both patients and manufacturers

Access to small physician practices is the goal

TraceLink will oppose the motion


Company emphasizes its clinical-to-commercial scope

Adds to company’s Forecasting Practice


Move unites pharma efforts with larger healthcare network
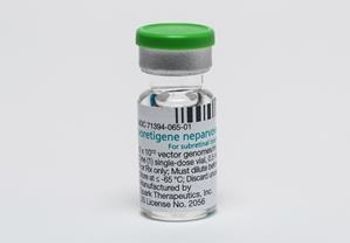
Therapy addresses a rare inherited form of vision loss

$5-million settlement resolves six-year-old issue

Washington Post and 60 Minutes question McKesson’s 2017 $150-million fine