
The deal provides the CDMO with ownership of the Lexington facility, allowing for late-phase and commercial gene therapy development and manufacturing of a branded severe Hemophilia B product.

The deal provides the CDMO with ownership of the Lexington facility, allowing for late-phase and commercial gene therapy development and manufacturing of a branded severe Hemophilia B product.
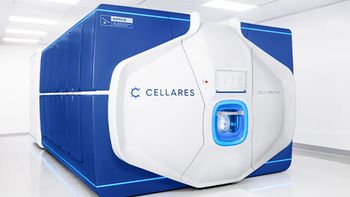
Following the evaluation, the company will decide whether it wants to move forward with the IDMO’s automated, end-to-end, cell therapy manufacturing platform.
The company’s latest investment—featuring new cGMP suites at its Devens, MA plant—is valued at $30 million.

The North Carolina project adds 1.4 million square feet of dedicated production space for aseptic manufacturing and finished production processes.

The company acquires Alimera Sciences for $381 million upfront, while establishing its interest in ophthalmology.

Mark Bouck discusses PharmAlliance’s latest acquisitions, while reiterating its goals surrounding health economics and outcomes research.
The acquisition of the 87,000 square-foot Camden, MD plant provides the CDMO with the ability to offer clinical and commercial non-viral aseptic fill-finish services on four fill lines.
Deal will target neurological diseases, featuring an initial payment of $42 million for Ascidian and up to $1.8 billion in milestone payments/royalties.

An analysis of the general medicine portion of the pharmacy market amid ensuing challenges.

Financial commitment will go toward expanding the CDMO’s integrated antibody-drug conjugate services.

The deal grows it pipeline, powered by panCAR programs in autoimmune disease and oncology.

With the acquisition, Merck’s US and Canada life science business aims to further increase its viral vector manufacturing capabilities.
The deal features felzartamab, an investigational anti-CD38 monoclonal antibody, whose clinical outcomes have shown potential to tackle various immune-mediated diseases.

The agreement is expected to focus on cell manufacturing processes, with potential for sharing tech with academia, startups.
The facility, company says, will be its first that covers end-to-end ADC production.
The company increases its selection of atopic dermatitis treatments in the process.

Leaders in the space discuss aspects of experimental drug support and more at this year’s US Pharma and Biotech Summit.
A cohort study investigates how the use of a murine chimeric monoclonal antibody biosimilar—as opposed to the original biological drug—affects healthcare expenditures.

IQVIA report explores the latest spending tendencies in the United States, while providing additional clarity as to why COVID-19 vaccines and therapeutics continue to experience a decline in volume.

The acquisition, the parties say, will benefit pharma and biotech in the clinical and commercial stages.
Collaboration will be oncology based—centered around allogeneic CAR-T cell therapy product candidates—and will provide Poseida with up to $550 million if certain milestones are met.

Per the US strategic partnership agreement, the adalimumab high-concentration interchangeable biosimilar will be distributed under the Quallent Pharmaceuticals private label.

Study explores how the Inflation Reduction Act’s Drug Price Negotiation Program and its maximum fair prices impact incentives for investments in post-approval activity.
The merger exponentially grows ONO’s oncology pipeline.

The complex will research solutions for manufacturing antibodies, mRNA applications, and more.