
Joins network of manufacturers contributing efforts to increase supply

Linkage to the company’s SensiWatch logistics-tracking technology is built-in

The latest people news happenings over the past month

Alliance with Jointown comes in time for Covid-19 vaccine storage and distribution efforts

Agency allows a stability budget of up to 2 weeks at -25°C

Joins UK consortium to help efficiently manufacture oral solid dosage medication

Deals provide Andlauer Healthcare Group full Skeleton Canada ownership and 49 percent stake in US branch

Country's Ministry of Public Health chooses cannabinoid pharma company as its medical supplier

Supply chain management and logistics company boosts its US presence

Company says it will be able to produce an estimated 12 million doses per month

While clock now ticking for life sciences companies to comply with the new data standards, opportunities in the offing to simplify manufacturing and the supply chain

Acquisition is 3PL provider's largest one yet

Acquisition grows Antares’ track-and-trace presence
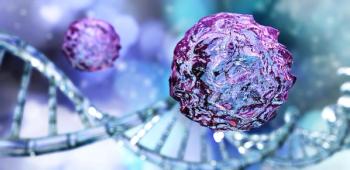
The importance of thinking strategically about the infrastructure and foresight needed to scale up CGTs—not as treatments of last resort, but first choice

Strengthens the CRO's scientific capabilities in cell and gene therapy space

Lab development project features 9,000-square-foot expansion

Combine their expertise in immunology and infectious diseases to speed up development of mAbs for influenza

Between both Covid-19 vaccines, an additional 350 million doses are being sent to Europe this year

Acquires Scientific Commercialization, a medical affairs consulting and analytics business

Authorization should activate distribution of hundreds of millions of doses to countries involved with COVAX effort

PBM's own specialty pharmacy unit adds CareMetx Health, growing its coverage base to the eastern US

Deal will help expand its pharma presence

Becomes a partner in UK consortium focused on enabling new and disruptive manufacturing technologies

UK cell and gene therapy location expected to be cGMP-ready by mid-2022

Will help biomed startups in furthering oncology R&D

There are currently 100,000 doses standing by

Results address concerns surrounding bulk passive pharma packaging

Survey of orphan drug commercial operation leaders gives glimpse into market challenges

As efficiencies increase, company aims to cut time from 110 to about 60 days, according to a published report