
MarkMonitor updates its surveillance surveys against counterfeiting and drug diversion

MarkMonitor updates its surveillance surveys against counterfeiting and drug diversion

New healthcare proposals re-open talk of drug importation

Government at all levels are trying to mobilize to address the crisis

Friction continues with Express Scripts as well

Advisory program makes data reporting more uniform

Latest 'delegated regulation' of the Falsified Medicines Directive is promulgated
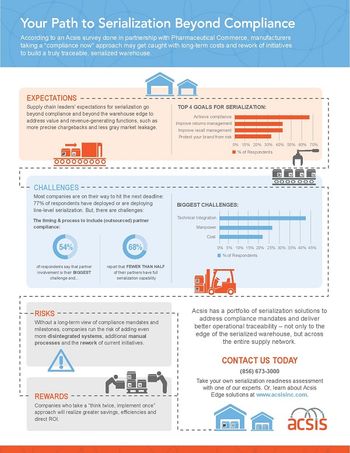
Acsis polls industry sentiment; TraceLink touts doubling of revenue

TGaS Advisors expands its roster of services
Good growth is occurring in the vaccine business, despite the hurdles of public perceptions and public-health funding shortfalls


Office of Prescription Drug Promotion finds little to complain about

'Average Manufacturer Price' is now a mostly settled matter

Industry is doing better at filing studies, but the agency is falling behind reviews, says GAO

Company partners with Teikoku for the latter's first marketed oncology product, and earns a milestone payment from Teva

'European companies will not be able to follow the exact path to operational compliance as their US counterparts,' says IMS Health survey

Quality control remains a problem among compounding pharmacies, but bigger players are entering the field

Promotional content produced for, or derived from, digital media presents its own management and compliance complexities

Thousands of poorly regulated websites are targeted

The growing number of certifications has become a competitive issue for SPPs bidding for Pharma's business

It's still early days in making medical marijuana--and cannabis-based pharmaceuticals--part of normal dispensing

Roughly 40% are for rare diseases; PD-1 oncolytics capture industry attention


GS1 Healthcare US publishes updated track-and-trace guidance; vendors revamp their offerings

Pharma companies will need to do more than rely on pharmacies' adjudication of claims