
The Pharmaceutical Distribution Security Alliance proposes an industry governance process

The Pharmaceutical Distribution Security Alliance proposes an industry governance process

Trace Histories employs blockchain technology

Combination of a pioneering traceability provider and a leading consulting firm

Rupture happens at a crucial time in Washington for the pharma industry

Presidential campaigns only magnify an already intense debate

Regulation enables better coordination of where opioids are going

Agency tries to get a handle on cross-border trade

Issues include apparently diverted opioids and mislabeled medications

Will restricting use of rebates in federal programs carry over to commercial insurance?

Senate and House committees begin the legislative process for addressing drug pricing
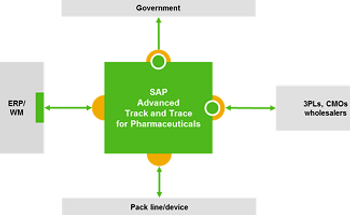
Nov. 27, 2018, was to be a red-letter day in the 10-year program to implement an end-to-end pharma traceability system. Did industry meet the deadline?

Programs provide copay and other financial assistance

$360-million settlement involved assistance funneled through a charity

HFAP builds out from its hospital-accreditation business

More-complete information is desirable on some applications

New survey finds weaknesses in patient privacy, funding oversight

Nov. 28 marks the first day when the US industry meets the DSCSA serialization mandate

The battle between pharma and PBMs over copays intensifies

Five Rivers Rx’s NavigateSOM software analyzes suspicious order monitoring

CDC data point to a three-month decline in overdose deaths

November 2018 serialization deadline is looming—and FDA will not provide another extension

$700,000 fine to benefit New York State residents

Not compensating for speaker programs and sponsored meetings has affected marketing, says the firm

Accreditation status is both an entry ticket and a competitive measure for specialty pharmacies to attract manufacturer attention

The year-old-plus litigation highlights the value of customer master data