
AAPA is positioning itself to provide alternative certification

AAPA is positioning itself to provide alternative certification

WDSrx expands controlled substances storage


The growing compliance requirements for distribution call for a streamlined approach

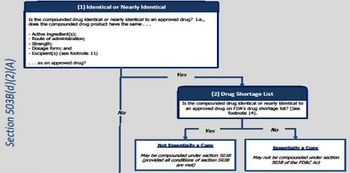
New guidance restricts some compounding to other than “essentially copies” of approved products

check on your doc, a company or a form of compensation

Pharma sales practices get raked over again—to what end?

Case falls apart as a 'skeptical' judge calls the company 'factually innocent'

Feds contend that FedEx knowingly performed illegal drug deliveries

rfXcel’s Traceability Platform becomes part of Verizon’s ThingSpace

New physician’s form is supposed to reduce the complexity of obtaining investigational drugs

Ensuring Patient Access and Effective Drug Enforcement Act (S. 483) calls for better industry-government coordination

Labels will have a combination of reference-product and biosimilar-specific information

An all-compounding "manufacturer" has big plans for growth

Announces a new version of its "platform as a service," with connectivity to Apache Spark

Ophthamology tops the breakout by physician specialty

Sample distribution management, payments reporting and sales-rep training

The anatomy of an optimized sampling supply chain

Pharmaceutical Commerce's annual Cold Chain Sourcebook projects 52% growth between 2014 and 2020

Database now covers 35 countries and 19 million HCPs
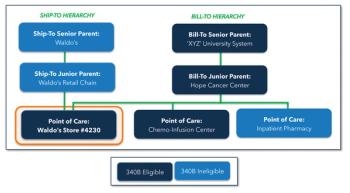
Discerning how and where products are discounted is an expensive headache for manufacturers

Looking at 'cost' and 'value' under different evaluation processes

MarkMonitor updates its surveillance surveys against counterfeiting and drug diversion

New healthcare proposals re-open talk of drug importation