
Upstart organization seeks to create another pathway for acceptance by regulators and pharmacy benefit managers

Upstart organization seeks to create another pathway for acceptance by regulators and pharmacy benefit managers

Certification of its Itasca, IL central control tower

Trading partners will have until November 2020 to get their IT systems into operation

A critical time for carrying DSCSA forward

Provider offers absorbents to manage hazardous drug cleanup
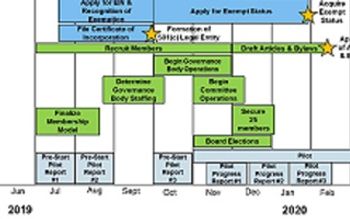
Updated prospectus proposes membership rules, budget and leadership


A changed look but not a changed direction

HHS Azar announces a plan to make a plan, roughly in time to be a talking point in next year’s national election

HDA Research Foundation highlights constraints and additional costs of reimportation

News follows court ruling against advertising retail drug prices

CDER activity is one of the few exceptions

Cross-contamination and missing documentation are cited in warning letters

Meanwhile, federal enforcement actions over improper patient assistance continue to arise



Investigative journalist Katherine Eban returns with an indictment of generic drug quality

Association will promote its members' value to the public via digital advertising and publishing

Rochester Drug Cooperative’s former CEO, Laurence Doud, is arrested

Counterfeit Xanax (alprazolam), plus meth and other controlled substances, were sold on the dark web

A final rule is still in the works at HHS, but some manufacturers are testing the inclusion of prices in TV ads

The Big Three, plus Rochester Drug, face civil penalties over opioid distribution

The Pharmaceutical Distribution Security Alliance proposes an industry governance process

Trace Histories employs blockchain technology

Combination of a pioneering traceability provider and a leading consulting firm