
Founding Partner of Curatio Scientia speaks on her upcoming panel at Trade & Channel Strategies 2023.

Founding Partner of Curatio Scientia speaks on her upcoming panel at Trade & Channel Strategies 2023.

AstraZeneca's deal to acquire Icosavax is expected to bolster AstraZeneca’s Vaccines & Immune Therapies late-stage pipeline with IVX-A12, a potential first-in-class vaccine for both respiratory syncytial virus and human metapneumovirus.

Bristol Myers Squibb strikes deal with SystImmune for the rights to codevelop and sell a potentially first-in-class EGFRxHER3 bispecific antibody-drug conjugate that has shown promise in non-small cell lung cancer and breast cancer.
Phase III INTerpath-002 trial will evaluate the novel individualized neoantigen therapy V940 (mRNA-4157) with Keytruda (pembrolizumab) as an adjuvant treatment for patients with non-small cell lung cancer.

Adults and children aged 1 year and older are eligible for the IV formulation of Cresemba for invasive aspergillosis and invasive mucormycotic, whereas the prescribed capsule is only indicated for adults and children 6 years of age and older.

The rise in demand represents the third consecutive month of year-on-year growth.
FDA approves bluebird bio’s Lyfgenia and Vertex Pharmaceuticals' and CRISPR Therapeutics’ Casgevy for the treatment of sickle cell disease, which affects approximately 100,000 people in the United States.

Study analyzes and predicts how conditions in research and development can be predicted via computer simulation.
PrEPVacc tested two different combinations of vaccines to determine whether either can prevent HIV infection in populations at risk of acquiring the virus.
An independent Data Monitoring Committee found that Keytruda in combination with chemotherapy followed by Keytruda plus Lynparza did not produce an improved overall survival benefit vs. Keytruda in combination with chemotherapy followed by Keytruda plus a placebo in patients with metastatic squamous non-small cell lung cancer.

Novartis anticipates Fabhalta to be available in the United States in December for the treatment of paroxysmal nocturnal hemoglobinuria.

Eli Lilly and Company's obesity drug Zepbound (tirzepatide) has a list price of $1,059.87, which is approximately 20% less than the 2.4 mg semaglutide injection indicated for weight loss.

Johnson & Johnson’s TAR-200 has a novel targeted releasing system that allows for a controlled release of gemcitabine in patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer.
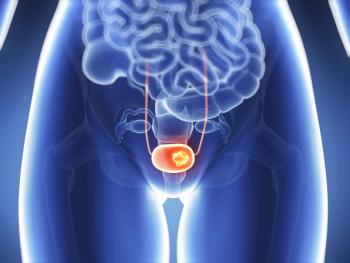
Opdivo (nivolumab) plus cisplatin-based chemotherapy shows improved survival benefits in the first-line treatment of adults with unresectable or metastatic urothelial carcinoma.
Critical factors need to be addressed for pharma stakeholders to realize the full potential of generative artificial intelligence.
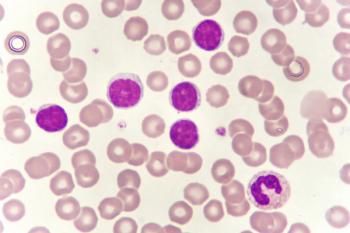
Jaypirca (pirtobrutinib) is indicated for adults with chronic lymphocytic leukemia or small lymphocytic lymphoma who received at least two prior lines of treatment with a Bruton tyrosine kinase inhibitor and a BCL2 inhibitor.
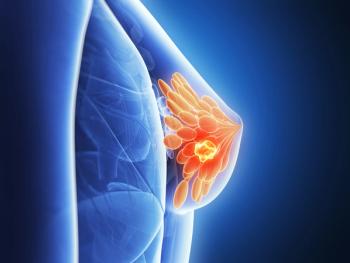
Ongoing clinical trials are evaluating zotatifin (eFT226) plus Faslodex (fulvestrant) and Verzenio (abemaciclib) for the treatment of ER–positive, HER2-negative advanced or metastatic breast cancer.
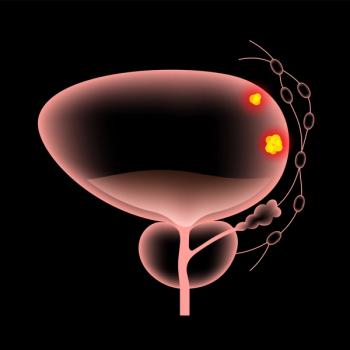
In clinical trials, Keytruda plus Padcev lowered the risk of disease progression or death by 55% compared with chemotherapy in adult patients with locally advanced or metastatic urothelial carcinoma.

Sphingosine-1-phosphate receptor modulator is designed to improve leukemia-free survival by increasing graft-versus-leukemia response.
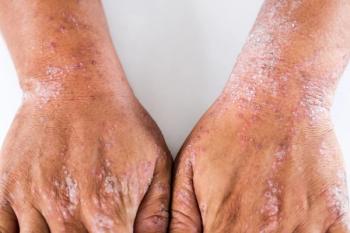
In clinical trials, roflumilast cream demonstrated rapid and sustained improvement in the signs and symptoms of atopic dermatitis in patients 6 years of age and older.

The companies will partner up to design and implement solutions that support each other's business efforts.

In clinical trials, KarXT (xanomeline-trospium) demonstrated a combination of strong tolerability and clinically meaningful symptom reduction in adult patients with schizophrenia.

CDC finds administration rates for influenza antiviral medication was lower than during pre–COVID-19 pandemic flu seasons.

Biden Administration invokes Defense Production Act to facilitate domestic manufacturing of essential medicines.

Studies explore how both the UK and EU are handling these ongoing supply chain challenges.

The approval of Ogsiveo represents an important therapeutic advance for patients with progressing desmoid tumors requiring systemic treatment.
Investigation notes a need for greater transparency in 340B program revenue and strengthened enforcement authority for the Health Resources and Services Administration.

The designation—which ranks the fastest-growing companies in North America—is powered by AeroSafe’s 477% growth rate.
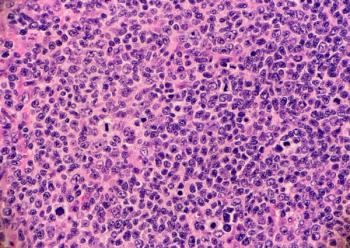
AbbVie and Genmab will share commercial responsibilities in the United States and Japan for epcoritamab-bysp (Epkinly) for the treatment of adults with follicular lymphoma that is relapsed or refractory following treatment with two or more therapies.