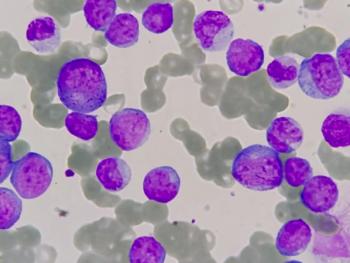
First-in-Class Novel Therapy for Chronic Myelomonocytic Leukemia Granted FDA Orphan Drug Designation
Immune-Onc Therapeutics’ IO-202 is currently being analyzed as a monotherapy and in combination for patients with relapsed/refractory acute myeloid leukemia with monocytic differentiation or chronic myelomonocytic leukemia.