
A cohort study investigates the impact of Senate Bill 2994A and answers the question: does the repeal of school-entry nonmedical vaccination exemptions result in a rise in school vaccinations?

A cohort study investigates the impact of Senate Bill 2994A and answers the question: does the repeal of school-entry nonmedical vaccination exemptions result in a rise in school vaccinations?
UV1 is an off-the-shelf vaccine that has demonstrated a survival benefit in combination with ipilimumab (Yervoy) and nivolumab (Opdivo) in patients with unresectable malignant pleural mesothelioma.
The acquisition expands Merck’s portfolio in the veterinary pharma space.
The deal is expected to help boost supply of Wegovy.

Pharma Commerce travels to Orlando for a pulse-check on this fast-evolving landscape in patient care.

And the need to boost awareness of this critical healthcare resource.

An analysis of the two main new models for pharmacy—unique direct—to-consumer for brands and cash pay for generics—and what members of industry think about them.
An analysis conducted by RAND Corporation determines that majority of new Rx medications are first sold in the United States before making their debut in other countries.
New reservation program for Beyfortus (nirsevimab-alip) seeks to improve access to the monoclonal antibody that is FDA-approved to protect against respiratory syncytial virus-associated lower respiratory tract disease.

A dive into this market reveals that the latest numbers yield promising results—a global value of a worldwide $1.6 trillion—but it will continue to be attributed to past, present, and future healthcare spending.

Allarity reached an agreement with Novartis in 2018 to license dovitinib, a small molecule multi-tyrosine kinase inhibitor under evaluation for the treatment of renal cell carcinoma.
Treatment with the novel engineered T-cell receptor produced an overall response rate of about 39% in patients with heavily pretreated synovial sarcoma.

Leading experts in the sector explore the value of third-party logistics providers when it comes to fortifying pharmaceutical supply chains.
BST02 is a novel cell-based immunotherapy currently being evaluated in a Phase I trial for the treatment of all types of liver cancer.
Health Affairs study investigates the consequences of patient medication abandonment surrounding oral HIV pre-exposure prophylaxis.

In order to provide both profitability and medication access, a balancing act is required.
Duplicate rebates have resulted in revenue leakage for manufacturers.
DELFI-TF assay shows promise in evaluating treatment response and resistance to immunotherapies in patients with advanced cancers.
Rusfertide could potentially be a first-in-class, transformational treatment for polycythemia vera.
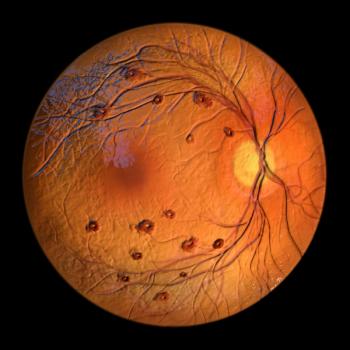
Vabysmo, the first bispecific antibody approved by the FDA for ocular conditions, produced significant drying of retinal fluid, which is often associated with blurry vision.

Per the deal, the company establishes Regeneron Cell Medicines, while also providing it with access to the collection’s full development and commercialization rights.
Biogen and partner Eisai will shift focus to advancing Leqembi, the first anti-amyloid beta treatment for Alzheimer disease to be granted traditional approval by the FDA.

Under the DSCSA law, wholesale distributors, re-packagers, dispensers, and other third-party logistics providers must implement interoperable and electronic tracing of prescription products at the package level.

In an interview with Pharma Commerce associate editor Don Tracy, Lynn Kirkpatrick, PhD, CEO, Ensysce Biosciences, discusses the FDA's Breakthrough Therapy Designation for PF614-MPAR, which focuses on protecting patients from drug overdoses.
As a result, time to treatment for the brand Gilead product is expected to increase from 16 to 14 days.
FDA to evaluate Darzalex Faspro with bortezomib, lenalidomide, and dexamethasone for induction and consolidation treatment and with lenalidomide for the maintenance treatment of adults newly diagnosed with multiple myeloma who are eligible for autologous stem cell transplant.
FDA to evaluate Padcev (enfortumab vedotin) with Merck’s Keytruda (pembrolizumab) for the first-line treatment of adults with previously untreated locally advanced or metastatic urothelial cancer.

The pharmaceutical packaging company unveils a new siliconization line at its SQLM plant in France.
New England Journal of Medicine study provides a breakdown of how hospitals—especially those that qualify for discounts under the federal 340B Drug Pricing Program—are able to make a profit off insurer pharmaceutical expenditures.

Breyanzi is a CD19-directed CAR T-cell therapy approved to treat multiple hematologic cancers.