The pTau217 antibody helps pinpoint pathological changes related to the disease.

The pTau217 antibody helps pinpoint pathological changes related to the disease.

The latest news for pharma industry insiders.

Momentum from the previous two years will propel the sector into the rest of 2024, says AM Best analysis.

The purchase boosts the parties’ sterile liquid manufacturing capabilities.

The latest news for pharma industry insiders.

A JAMA Network Open study dives into how the utilization of a digital patient portal changed during the COVID-19 pandemic, while also exploring ways that multimorbidity and socioeconomic disparities affected those statistics.

With its aims to protect healthcare consumers against the dangers of counterfeit, contaminated, stolen or otherwise harmful drugs, the DSCSA represents a significant step forward in securing the pharmaceutical supply chain.

The offering reportedly helps avoid product quarantine.

A cohort study investigates a potential correlation between the stresses caused by Mother Nature and mental health.
IPX203—an oral formulation carbidopa/levodopa extended-release capsules —would expand Zambon’s neurology portfolio.

Rob Besse offers a breakdown of the third-party logistics (3PL) process, including lessons that these providers have learned coming out of the pandemic.

In an interview with Pharma Commerce Associate Editor Don Tracy, Corey Ford, VP, Reimbursement & Policy Insights, Cencora, offers his thoughts on where the Inflation Reduction Act will be five to 10 years from now.

A Carrum Health/PWC report surveys benefit managers spanning various self-insured employers in an effort to determine ways to improve the cancer treatment experience, especially from a cost perspective.

An MDVIP study reports that 61% of people feel hassled by the current structure.

In an interview with Pharma Commerce Associate Editor Don Tracy, Corey Ford, VP, Reimbursement, Policy Insights, Cencora, provides a look at his recent webinar focused on the implications of the Inflation Reduction Act.
An investigation evaluates whether SNAP benefits can also influence hypertension patients’ lack of medication adherence.
Compared with piperacillin/tazobactam, Exblifep (cefepime/enmetazobactam) showed noninferiority and superiority in a clinical cure and microbiological eradication of complicated urinary tract infections.

Current production of Qdenga will increase to 50 million doses a year.
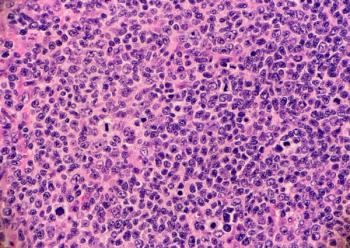
Epkinly (epcoritamab-bysp), a subcutaneously administered, T-cell engaging, bispecific antibody, was previously granted Breakthrough Therapy Designation for the treatment of patients with relapsed or refractory follicular lymphoma following two or more prior lines of therapy.

A cohort study explores the association of Medicare Advantage enrollment with post-acute care use, along with patient outcomes among retired state employees.

The company’s cloud technology services aim to assist healthcare and pharma with AI integration.

Xolair was approved last week to reduce allergic reactions from exposure to one or more food allergens in individuals over 1 year of age with IgE-mediated food allergy.
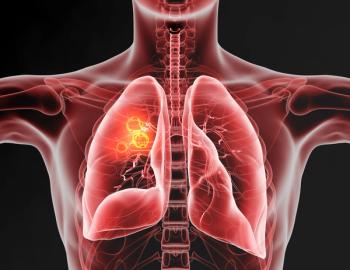
Bayer's BAY 2927088 is under evaluation to treat unresectable or metastatic non-small cell lung cancer with tumors harboring activating HER2 mutations.

A cross-sectional study investigates how this supply chain link helps increase Medicare Part D spending on self-administered specialty drugs.

SKYTyphoid, a single-dose typhoid conjugate vaccine jointly developed by SK bioscience and the International Vaccine Institute, showed a positive immunogenicity and safety profile across age groups.

The CRO’s FortreaRx doubles in size, including a boost in cold chain storage, while also continuing to improve its patient access services.

Trial data show Dupixent is the first and only novel biologic drug to significantly improve lung function and reduce severe acute exacerbations in adults with uncontrolled chronic obstructive pulmonary disease.

The latest news for pharma industry insiders.

The annual report published by IQVIA examines movement surrounding drug launches and new clinical trials, during the post-pandemic era.
A cohort study investigates how a change in clinical guidelines, an expanded label, and reduction in drug prices can impact the use of PCSK9 inhibitors as a treatment for atherosclerotic cardiovascular disease.