
Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) was found to improve overall survival in metastatic pancreatic adenocarcinoma.

Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) was found to improve overall survival in metastatic pancreatic adenocarcinoma.

Properly supporting market access for a newly launched product with a single indication is challenging. Doing so for multiple indications, or supporting subsequent indications, can have a compounding effect on the degree of difficulty for getting everything right.
Analysis explores where drug prices are derived from, and whether a holistic societal approach can help determine these products’ true value.
Bruce Phelan of Blue Fin discusses Novo Nordisk's purchase of Catalent manufacturing sites to increase its ability to meet the demand for the popular weight loss drug Wegovy.
Examination evaluates investigational stem cell interventions, including how the states have implemented their own policies when it comes to their uses.

The analysis notes that 60% of respondents were setting targets based on AI’s ability to either cut costs or boost productivity.

Roche and Repare came to terms on a deal in June 2022 to develop and commercialize camonsertib, which included a $125 million upfront payment and could have reached up to $1.2 billion based on clinical, regulatory, commercial, and sales milestones.
BXCL701 combined with Keytruda (pembrolizumab) is being evaluated for patients with metastatic castration-resistant prostate cancer with small cell neuroendocrine and adenocarcinoma phenotypes.
Cohort study explores value-based insurance designs, while also determining how one’s place of residency can potentially play a role.
In clinical trials, bepirovirsen was found to recognize the RNA used by hepatitis B virus to replicate in infected liver cells.
Eohilia, a corticosteroid developed specifically to treat eosinophilic esophagitis, is comprised of a novel formulation of budesonide taken twice daily for 12 weeks in patients 11 years of age and older.

According to AbbVie, ImmunoGen's entire oncology portfolio could drive long-term revenue growth, giving the company a potential multi-billion dollar drug that is already on the market.

Researchers explore the proportion of approved drugs in the Canadian marketplace that utilize manufacturer-sponsored patient support programs, along with the types of medications that are more likely to do so.

Technavio analysis notes that an increase drug patent expirations could be a contributing factor.
New collaboration seeks to take a significant step in the development of targeted treatments for solid tumors and an expansion of opportunities in the growing antibody-drug conjugate market.

A dive into the intricacies of how to ensure a long-running event stays fresh and relevant to the needs of the pharmaceutical industry.

Investigators examine the validity of phosphodiesterase type 5 inhibitors for erectile dysfunction, such as Viagra, being repurposed for the treatment of Alzheimer's disease.
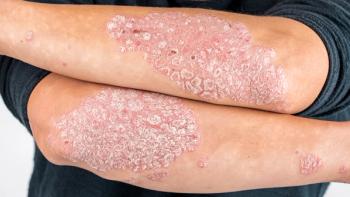
JNJ-2113, a novel, oral IL-23–receptor antagonist peptide showed significant efficacy in patients with moderate-to-severe plaque psoriasis compared with placebo.
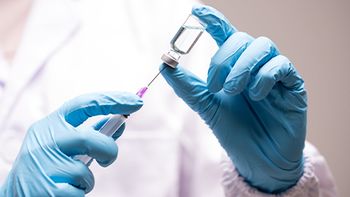
A study investigates the effectiveness of bivalent mRNA COVID-19 vaccines against the Omicron variant among participants 5-17 years of age.

The FDA placed a full clinical hold on magrolimab for hematologic cancers after data showed the drug in combination with azacitidine plus Venclexta increased the risk of death related to infections and respiratory failure in patients with acute myeloid leukemia.

Data suggest that dosing of MariTide may be effectively tapered down and the drug may be taken less frequently over time compared with GLP-1 agonists such as Wegovy and Zepbound.
Trial for the treatment of ATTR-CM demonstrated an 81% absolute survival rate, along with an observed mean annual CVH frequency of 0.29.

The pharma powerhouse is investing $300 million in a plant that could be vital for the future cancer trials.

Phase III CheckMate -77T trial data show statistically significant improvements in event-free survival with Opdivo (nivolumab) plus chemotherapy followed by surgery and adjuvant Opdivo in the treatment of resectable stage IIA to IIIB non-small cell lung cancer.

DB Schenker, JAS, Medista, MSC Air Cargo, OnAsset Intelligence, and xpd global round out the latest additions.

Cohort study dives into whether low income subsidy losses are associated with disability status, age, and race and ethnicity.
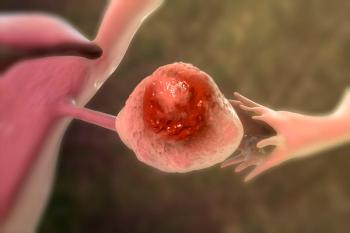
BioNTech SE and Duality Biologics Co's BNT325/DB-1305 is a next-generation antibody-drug conjugate that targets the TROP2 protein, which is overexpressed across various tumor types.
Arexvy was the first FDA-approved respiratory syncytial virus (RSV) vaccine to prevent lower respiratory tract disease caused by RSV in individuals 60 years of age and older.

The air cargo market numbers close out the year slightly below 2022’s performance.

The February issue is centered around third-party logistics providers, including new dynamics and future trends that affect this key supply chain link.