
The partnership revolves around meeting various agency standards and industry requirements pertaining to the drug development and manufacturing lifecycle.
FDA Accepts, Grants Priority Review to NDA for Vorasidenib in IDH-Mutant Gliomas
Krazati Combination for Advanced, Metastatic Colorectal Cancer Granted FDA Priority Review

The partnership revolves around meeting various agency standards and industry requirements pertaining to the drug development and manufacturing lifecycle.

Xolair (omalizumab) was previously granted FDA Priority Review status and Breakthrough Therapy Designation to prevent severe allergic reactions after accidental exposure to one or more foods in people with allergies.
Tepmetko (tepotinib), an oral MET inhibitor, was granted accelerated approval in 2021 for metastatic non–small cell lung cancer harboring MET exon 14 skipping alterations.

There were no safety or efficacy issues identified in the FDA complete response letter for Lymphir, with no additional trials required.
Patients with severe frostbite who were administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation.
Augtyro was previously approved in November 2023 to treat locally advanced or metastatic ROS1-positive non-small cell lung cancer.

Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) was found to improve overall survival in metastatic pancreatic adenocarcinoma.
Examination evaluates investigational stem cell interventions, including how the states have implemented their own policies when it comes to their uses.
BXCL701 combined with Keytruda (pembrolizumab) is being evaluated for patients with metastatic castration-resistant prostate cancer with small cell neuroendocrine and adenocarcinoma phenotypes.
In clinical trials, bepirovirsen was found to recognize the RNA used by hepatitis B virus to replicate in infected liver cells.
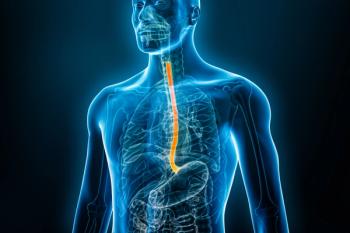
Eohilia, a corticosteroid developed specifically to treat eosinophilic esophagitis, is comprised of a novel formulation of budesonide taken twice daily for 12 weeks in patients 11 years of age and older.

Technavio analysis notes that an increase drug patent expirations could be a contributing factor.

The FDA placed a full clinical hold on magrolimab for hematologic cancers after data showed the drug in combination with azacitidine plus Venclexta increased the risk of death related to infections and respiratory failure in patients with acute myeloid leukemia.

Phase III CheckMate -77T trial data show statistically significant improvements in event-free survival with Opdivo (nivolumab) plus chemotherapy followed by surgery and adjuvant Opdivo in the treatment of resectable stage IIA to IIIB non-small cell lung cancer.
BioNTech SE and Duality Biologics Co's BNT325/DB-1305 is a next-generation antibody-drug conjugate that targets the TROP2 protein, which is overexpressed across various tumor types.
Arexvy was the first FDA-approved respiratory syncytial virus (RSV) vaccine to prevent lower respiratory tract disease caused by RSV in individuals 60 years of age and older.

A cohort study investigates the impact of Senate Bill 2994A and answers the question: does the repeal of school-entry nonmedical vaccination exemptions result in a rise in school vaccinations?
UV1 is an off-the-shelf vaccine that has demonstrated a survival benefit in combination with ipilimumab (Yervoy) and nivolumab (Opdivo) in patients with unresectable malignant pleural mesothelioma.

Allarity reached an agreement with Novartis in 2018 to license dovitinib, a small molecule multi-tyrosine kinase inhibitor under evaluation for the treatment of renal cell carcinoma.
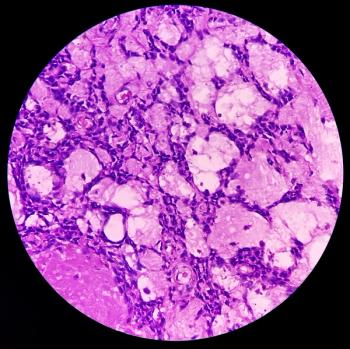
Treatment with the novel engineered T-cell receptor produced an overall response rate of about 39% in patients with heavily pretreated synovial sarcoma.
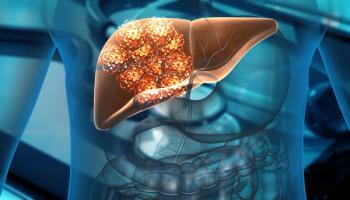
BST02 is a novel cell-based immunotherapy currently being evaluated in a Phase I trial for the treatment of all types of liver cancer.
Duplicate rebates have resulted in revenue leakage for manufacturers.

A look at the challenges that need to be addressed during the FDA stabilization period.
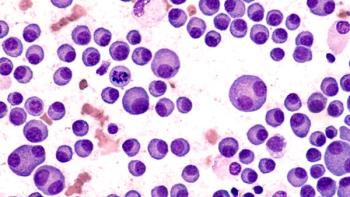
FDA to evaluate Darzalex Faspro with bortezomib, lenalidomide, and dexamethasone for induction and consolidation treatment and with lenalidomide for the maintenance treatment of adults newly diagnosed with multiple myeloma who are eligible for autologous stem cell transplant.
FDA to evaluate Padcev (enfortumab vedotin) with Merck’s Keytruda (pembrolizumab) for the first-line treatment of adults with previously untreated locally advanced or metastatic urothelial cancer.