
Novartis anticipates Fabhalta to be available in the United States in December for the treatment of paroxysmal nocturnal hemoglobinuria.


Novartis anticipates Fabhalta to be available in the United States in December for the treatment of paroxysmal nocturnal hemoglobinuria.

Johnson & Johnson’s TAR-200 has a novel targeted releasing system that allows for a controlled release of gemcitabine in patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer.
Opdivo (nivolumab) plus cisplatin-based chemotherapy shows improved survival benefits in the first-line treatment of adults with unresectable or metastatic urothelial carcinoma.
Jaypirca (pirtobrutinib) is indicated for adults with chronic lymphocytic leukemia or small lymphocytic lymphoma who received at least two prior lines of treatment with a Bruton tyrosine kinase inhibitor and a BCL2 inhibitor.
Ongoing clinical trials are evaluating zotatifin (eFT226) plus Faslodex (fulvestrant) and Verzenio (abemaciclib) for the treatment of ER–positive, HER2-negative advanced or metastatic breast cancer.
In clinical trials, Keytruda plus Padcev lowered the risk of disease progression or death by 55% compared with chemotherapy in adult patients with locally advanced or metastatic urothelial carcinoma.
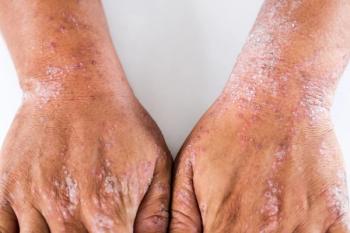
In clinical trials, roflumilast cream demonstrated rapid and sustained improvement in the signs and symptoms of atopic dermatitis in patients 6 years of age and older.

In clinical trials, KarXT (xanomeline-trospium) demonstrated a combination of strong tolerability and clinically meaningful symptom reduction in adult patients with schizophrenia.

The approval of Ogsiveo represents an important therapeutic advance for patients with progressing desmoid tumors requiring systemic treatment.
AbbVie and Genmab will share commercial responsibilities in the United States and Japan for epcoritamab-bysp (Epkinly) for the treatment of adults with follicular lymphoma that is relapsed or refractory following treatment with two or more therapies.

Alladapt Immunotherapeutics said the FDA Fast Track designation highlights the importance of developing a treatment for multiple food allergens.

Webinar Date/Time: Thu, Dec 14, 2023 11:00 AM EST

The FDA also approved a companion diagnostic test to detect the PIK3CA, AKT1, and PTEN alterations as part of the approval for AstraZeneca’s capivasertib (Truqap) plus fulvestrant (Faslodex) for HR-positive, HER2-negative locally advanced or metastatic breast cancer.
Enzalutamide (Xtandi) gets FDA approval to treat nonmetastatic castration-sensitive prostate cancer with biochemical recurrence at high risk for metastasis.

With new mandates right around the corner, there is an unwavering demand for pharma operations to perform at peak efficiency.
Pembrolizumab (Keytruda) plus fluoropyrimidine- and platinum-containing chemotherapy for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction cancer approved by FDA after being found to lower the risk of metastasis or death.
Bristol Myers Squibb’s repotrectinib (Augtyro) approved by FDA to treat locally advanced or metastatic ROS1-positive non-small cell lung cancer.
Patients with kidney failure administered chronic hemodialysis who received DefenCath showed a statistically significant lower incidence of catheter-related bloodstream infections.
Exparel was initially approved in 2011 for single-dose infiltration into a surgical site.
The Flowflex COVID-19 Antigen Home Test is the first over-the-counter rapid antigen test for SARS-CoV-2 to gain FDA 510(k) marketing clearance by the FDA.

Conference presentation explores groundbreaking topics impacting the pharma industry.
Fruquintinib (Fruzaqla) is a significant new therapy for patients who have had limited options to treat metastatic colorectal cancer.

Conference session discusses the drivers, practical considerations, success factors, and landscape for next-generation pharmacy innovation.

Ayala Pharmaceuticals’ AL102 is a gamma secretase inhibitor being studied to treat patients with desmoid tumors.
Surge acted as a precursor for the RSV-marketed drug landscape in 2023, says GlobalData.