
Supply Chain
Latest News
Latest Videos

More News

A keynote presentation describes how supply chain visibility should rely on digital capabilities.

A panel discussion breaks down ways for stakeholders to meet temperature-controlled operational demands pertaining to product pipelines.

The conference’s opening keynote case study shares ways to elicit change that not only results in business growth and productivity, but a boost in patient connection as well.

The parties aim to create an end-to-end digital thread that links research data all the way through to life sciences manufacturing.

In a move meant to encourage domestic production, the president’s executive order features a 10% baseline tax, expected to take effect April 9.
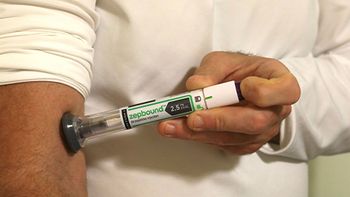
The telehealth company is offering Lilly’s branded form of tirzepatide—approved for weight loss—on its site for $1,899 a month, while Lilly also sells the GLP-1 via its LillyDirect platform.

CRYOPDP—centered around clinical trials, biopharma, and cell and gene therapies—augments DHL’s specialty pharma logistics expertise.

The move by the CDMO—who also houses a North American HQ in Indiana—expresses its interest in providing compliant storage and logistics solutions globally.

In the final part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Brad Stewart, BDO’s national life sciences co-leader, details the Biosecure Act, including the ways pharma companies are preparing for it, especially from a compliance standpoint.

The Beijing location represents the sixth worldwide.

In the fourth part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Brad Stewart, BDO’s national life sciences co-leader, addresses how AI can help support pharma leaders with managing their supply chains and addressing potential material shortages.

In the third part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Brad Stewart, BDO’s national life sciences co-leader, outlines the ways that tariffs disrupt pharma supply chains, along with how leaders can evaluate and prepare for the resulting challenges.

In the final part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Jason C. Foster, CEO of Ori Biotech, comments on how he envisions automation in the CAR-T manufacturing space playing a role in improving efficiency, reducing costs, and ultimately, expanding patient access.

The thermal packaging company not only bolsters its APAC presence—it also grows its influence in the life sciences’ temperature-monitoring logistics sector.

The CRDMO’s acquisition of the Baltimore plant is expected to boost its biologics presence.

In the second part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Brad Stewart, BDO’s national life sciences co-leader, outlines the internal constraints that CFOs are reporting as their biggest manufacturing obstacle, along with other hurdles they are encountering.

In the second part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Jason C. Foster, CEO of Ori Biotech, comments on the primary factors causing manufacturing bottlenecks in the space, along with how the industry can overcome them in order to scale up production.

The updated tax on Canadian imports will rise from 25% to 50%, taking effect March 12.

This link in the pharma supply chain is undergoing a major transformation propelled by technological advancements, regulatory changes, and evolving market dynamics, requiring industry leaders to adopt innovative strategies in order to remain competitive.

In the first part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Brad Stewart, BDO’s national life sciences co-leader, discusses how the tariffs impact the reshoring of manufacturing services, alongside the strategies that pharma leaders can use in order to start shoring up their supply chains.

The US president delays tariffs that comply with the 2020 USMCA for one month, with reciprocal tariffs still expected to go into effect by April 2.

In the final part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Tom Dorsett, CEO, RazorMetrics, describes how the software company’s solution can help deliver better patient outcomes and substantial savings for healthcare plans, employers, and members alike.

The agreement—powered by YASKAWA's Maholo robot—aims to address challenges in production and commercialization while offering access to academic and startup communities.
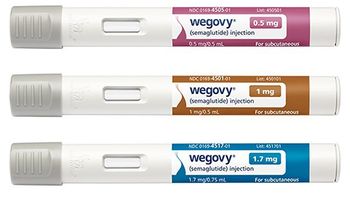
NovoCare will offer the GLP-1 injection to cash-paying patients for a discounted $499 per month.

Ron Lanton, Partner, Lanton Law, examines the legal and economic impacts of reciprocal tariffs on the pharma supply chain, including potential disruptions and increased drug costs.














