
Reimbursement is just one of the issues facing biosimilar commercialization in the US

Reimbursement is just one of the issues facing biosimilar commercialization in the US


The looming November 2017 deadline for US pharma serialization drives rapid implementation

RFID connectivity with dispensers and patients; energy efficiency for cold chain storage

New analysis paints a bleaker picture; implications for healthcare?

Two licenses have been granted; a third is in the works

IntrinsiQ Specialty Solutions and ION Solutions apply resources to the task

More capacity for frozen and refrigerated pharmaceuticals

A breathable version of the company’s Tyvek material minimizes humidity effects
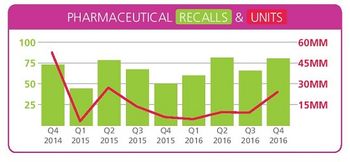
Q4 2016 Stericycle ExpertSolutions index counts 83 incidents, highest since 2014

The latest in cargo theft prevention, security and law enforcement activity

Q Products partners with RiskPulse, a meteorological forecaster

More hospital, but fewer practitioner deliveries

Shorter elapsed time in recent years shows encouraging improvement
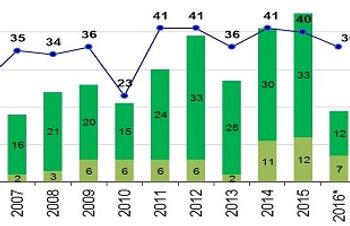
Only 20 new molecular entities passed muster this year, less than half of 2015’s total

Just released survey details lack of consensus and difficulties with current requirements

HDA’s latest Factbook tracks temperature-controlled distribution


Higher productivity and more complexity; but slow takeup of traceability requirements

Strong growth of existing clients and new product introductions are justification


Goldman Sachs jumps in, takes a board seat

Direct-to-patient trial supply and protocol management from Center Point

A standard, a user guidance and a business vocabulary; not for pharma applications alone

Bracket acquires CLINapps