Market Access
Latest News
Latest Videos

More News

An NEJM study explores how this loss—following Medicaid disenrollment—affects beneficiaries.

Andrew Witty resigns due to personal reasons, as Stephen Hemsley, chairman and former chief executive, will assume the position.

Ways to reimagine pharma’s operating model and KPIs for commercial success in an AI-driven future.

Doug Long, VP, industry relations, IQVIA, discusses how soaring demand for GLP-1 therapies is driving reimbursement challenges, shifting distribution models, and expanding the drugs’ potential into new therapeutic areas.

Fran Gregory, VP, emerging therapies, Cardinal Health, emphasizes the need for long-term outcomes tracking and robust health economic modeling to demonstrate the sustained value of advanced therapies and support future payment strategies.

Chip Parkinson, CEO, GiftHealth, explains how AI-powered data integration is bringing new hope to patients by revealing hidden access barriers, streamlining treatment pathways, and challenging outdated utilization management practices.

Tiara Green, president, Accessia Health, explains how empowering trusted local leaders and involving communities early are key to closing persistent gaps in preventive care and healthcare access.

Fran Gregory, VP, emerging therapies, Cardinal Health, discusses the evolving cell and gene therapy landscape, highlighting pipeline growth, cost challenges, and emerging therapeutic areas beyond oncology and hematology.

A seminar shares key observations and learnings from the first round of direct price negotiations.

Tiara Green, president, Accessia Health, highlights key themes from the upcoming discussion on how health equity, literacy, and social determinants are reshaping patient care—especially for those with chronic and rare conditions.

A session dives into the trends surrounding specialty drugs and therapies, along with providing an overview of the drug development landscape.
The interchangeability designation for Yuflyma, a biosimilar to Humira, may help to improve patient access and reduce costs.

In the third part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Chris O’Dell, provides an update on where the healthcare industry currently stands in terms of compliance with price transparency regulations, and what steps need to be taken to achieve full compliance.

In the second part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Chris O’Dell, Turquoise Health’s SVP of market solutions, discusses the biggest challenges healthcare organizations currently face in terms of price transparency.

The key strategies for reducing or eliminating post-prescription abandonment.

A look at the prevailing factors impacting treatment access in 2025.

How pharma manufacturers can steadily navigate GTN components and complexities when planning pharmacy benefit reimbursement.

Respective industry and government agendas could significantly reshape the US prescription drug market.

In the first part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Chris O’Dell, Turquoise Health’s SVP of market solutions, outlines the key provisions of the recently released executive order on healthcare price transparency.

How data-driven insights can help pharma companies balance their revenue management and innovation strategies—including resource allocation, pricing, and market access.

Jen Butler, chief commercial officer, Pleio, discusses how the company’s GoodStart programs help patients navigate specialty pharmacies.

Jonathan James, CEO, Hope Charities, discusses how, despite policy advancements, millions of people still face barriers to healthcare access.

A roundtable explores how patient access needs are being measured, while also discussing the value of affordability in decision-making.

The partnership focuses on enhancing the patient experience by providing greater affordability and access to specialty meds.
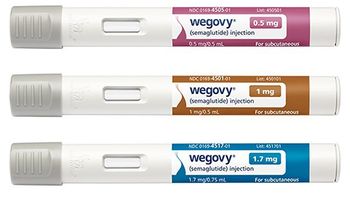
NovoCare will offer the GLP-1 injection to cash-paying patients for a discounted $499 per month.