
Market Access
Latest News
Latest Videos

More News

In the first part of her Pharma Commerce video interview, LeAnn Boyd, Liviniti’s founder and CEO, outlines the most critical root causes of rising healthcare and drug costs that the MFN executive order doesn’t address.

An exploration into how flawed incentives and contractual loopholes between manufacturers and pharmacy benefit managers played their part.

How practical is this form of pricing in the pharma setting?

In the final part of her Pharma Commerce video interview, Maggie McCullough, PolicyMap’s CEO, illustrates how data-driven insights can support PBMs and healthcare leaders in negotiating fairer drug prices?

In order to succeed in today’s outcomes-driven healthcare landscape, pharmaceutical companies must move from traditional sales models to strategic, measurable partnerships with integrated delivery networks (IDNs).

A cross-sectional study explores how full-benefit dual-eligible beneficiaries differ across MA plans with differing levels of Medicaid coordination and integration.
Marketed as BNT327, the PD-L1xVEGF-A bispecific antibody has the potential to become what the involved parties believe is a ‘foundational immuno-oncology backbone.’

Alice Valder Curran outlines the steps manufacturers should take to better prepare for upcoming executive actions on drug pricing and market access.

An NEJM study explores how this loss—following Medicaid disenrollment—affects beneficiaries.

Andrew Witty resigns due to personal reasons, as Stephen Hemsley, chairman and former chief executive, will assume the position.

Ways to reimagine pharma’s operating model and KPIs for commercial success in an AI-driven future.

Doug Long, VP, industry relations, IQVIA, discusses how soaring demand for GLP-1 therapies is driving reimbursement challenges, shifting distribution models, and expanding the drugs’ potential into new therapeutic areas.

A seminar shares key observations and learnings from the first round of direct price negotiations.

A session dives into the trends surrounding specialty drugs and therapies, along with providing an overview of the drug development landscape.
The interchangeability designation for Yuflyma, a biosimilar to Humira, may help to improve patient access and reduce costs.

The key strategies for reducing or eliminating post-prescription abandonment.

A look at the prevailing factors impacting treatment access in 2025.

How pharma manufacturers can steadily navigate GTN components and complexities when planning pharmacy benefit reimbursement.

Respective industry and government agendas could significantly reshape the US prescription drug market.

How data-driven insights can help pharma companies balance their revenue management and innovation strategies—including resource allocation, pricing, and market access.

A roundtable explores how patient access needs are being measured, while also discussing the value of affordability in decision-making.

The partnership focuses on enhancing the patient experience by providing greater affordability and access to specialty meds.
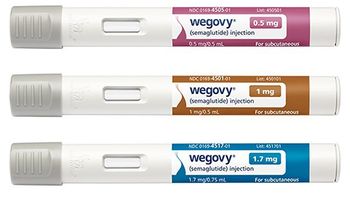
NovoCare will offer the GLP-1 injection to cash-paying patients for a discounted $499 per month.

In the final part of his video interview with Pharma Commerce Editor Nicholas Saraceno, Geoffrey Joyce, PhD, director of health policy at the Leonard D. Schaeffer Center for Health Policy & Economics at USC, describes how PBMs financially benefit from generics, and why a completely open generic market powered by cash would be beneficial.

The injection for the treatment of psoriatic arthritis—among other indications—is a biosimilar to Stelara.














