
The development and manufacturing plant will be constructed in Texas, and is expected to become operational by Q1 2025.

The development and manufacturing plant will be constructed in Texas, and is expected to become operational by Q1 2025.

The courts reversed certain key guidance documents issued by the Health Resources and Services Administration for the 340B Program, which raises uncertainty.

The new plant in Italy is expected to tackle the rise in biopharmaceutical material demand, specifically for GLP-1s.

A retrospective study explores the connection between adherence and racial/ethnic disparities among patients with Alzheimer’s disease, along with similar dementias.

The Netherlands plant is expected to provide solutions that can help further the development of advanced therapies.

In an interview with Pharma Commerce Editor Nicholas Saraceno, Lung-I Cheng, PhD, VP, Head of Cell & Gene Therapy Service Line, Cencora offers his thoughts on Cencora's inaugural ThinkLive Cell and Gene Therapy Summit.

Financial commitment will go toward expanding the CDMO’s integrated antibody-drug conjugate services.

These advancements continue to act as a key driver in helping to boost the security, efficiency, and innovation of global industries, include pharma.

According to the IATA, the streak continues—April represents the fifth consecutive month of positive development.

The Texas facility helps meet the cross-border logistics demand created by nearshoring.

The SSC strives to simply and add efficiencies to the scheduling technology used by the logistics sector.
Results from the CORE-001 clinical trial are expected to be presented at the American Society of Clinical Oncology (ASCO) 2024 Annual Meeting.
Among its benefits, the fund will provide up to $8,000 in medication copayment or insurance premium assistance.

The deal grows it pipeline, powered by panCAR programs in autoimmune disease and oncology.

With the acquisition, Merck’s US and Canada life science business aims to further increase its viral vector manufacturing capabilities.

The CDMO will run the active pharmaceutical ingredient pilot plant in Sandwich, UK, along with development laboratories.
The deal features felzartamab, an investigational anti-CD38 monoclonal antibody, whose clinical outcomes have shown potential to tackle various immune-mediated diseases.

The document reiterates the company’s commitment to ESG principles, including carbon neutrality by 2030.

The decision comes after success during formulation testing with convincing stability data.

The agreement is expected to focus on cell manufacturing processes, with potential for sharing tech with academia, startups.

In an interview with Pharma Commerce Associate Editor Don Tracy, Ron Lanton, Partner, Lanton Law offers his thoughts on the recent Federal Trade Commission (FTC) repeal of non-compete agreements, and how it could effect the pharma industry.
The facility, company says, will be its first that covers end-to-end ADC production.
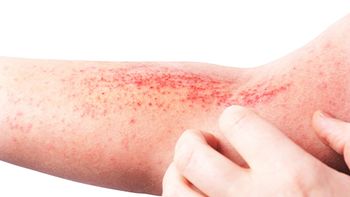
The company increases its selection of atopic dermatitis treatments in the process.

Leaders in the space discuss aspects of experimental drug support and more at this year’s US Pharma and Biotech Summit.

Chip Davis recaps the organization’s Distribution Management Conference and addresses distributors’ preparedness for DSCSA implementation.

The company has financially committed $13.1 million into the project.

The CDMO’s latest business venture reaffirms its commitment to the development of novel radiopharmaceuticals.

In an interview with Pharma Commerce Editor Nicholas Saraceno at LogiPharma Europe, Nick Porter, President, World Courier discusses mitigating supply chain risks, the future of artificial intelligence in pharma logistics, and more.

The company has financially committed more than $3.8 billion since the COVID-19 pandemic into keeping the medication and vaccine production process in France.

The development project adds an additional 157,500 square feet to the existing facility in the process.