
Business and Finance
Latest News

Latest Videos

Podcasts
More News

In the second part of her Pharma Commerce video interview, Tina Martinez, vice president and head of global products and solutions at Cencora, outlines a customer-first approach to partnering with pharmaceutical manufacturers—centered on active listening, early problem validation, and iterative piloting—to co-develop scalable solutions that support late-stage clinical programs through commercial launch.

The acquisition strengthens Yusen Logistics’ healthcare footprint across Europe, adding specialized transportation, warehousing, digital, and GDP-compliant capabilities to its regional and global network.

The South Korea–based CDMO acquires its first US manufacturing facility in Rockville, MD, expanding its global footprint and strengthening biologics capacity.

The acquisition strengthens CEVA’s global project logistics footprint, adding heavy-lift expertise, specialized engineering talent, and integrated solutions across key regions.

In today’s Pharma Pulse, Novartis and Moderna anchor a domestic manufacturing surge, while AI-driven biomarker discovery and physician well-being reshape the future of clinical care.

The pharmaceutical giant’s new 700,000-square-foot hub aims to onshore production of key medicines and strengthen the US drug supply chain.

In today’s Pharma Pulse, SOBI strengthens its gout franchise by acquiring Arthrosi Therapeutics for up to $1.5 billion, securing the Phase III asset pozdeutinurad, and much more.

A session explores how manufacturers can refine GTN strategy, strengthen pricing governance, and navigate increasingly complex distribution-channel economics, while maintaining both commercial performance and patient affordability.

BioCare's Ryan Cort outlines the pharmaceutical industry's "unprecedented sea change," driven by over $400 billion in anticipated revenue loss from generics and biosimilars losing exclusivity.

Welcome to Pharma Pulse, a Pharmaceutical Commerce podcast where we bring you the latest insights shaping patient access, supply chain/logistics, data & tech, and healthcare innovation. I’m your host, and let’s get into today’s headlines.

In today’s Pharma Pulse, the University of Maryland expands its medical class to combat physician shortages and much more.

Commercialization expert Bill Roth outlines the critical need for "adaptive capacity," detailing how accelerating government regulation and massive patent cliffs are forcing a complete overhaul of commercial strategies across various markets.

In today’s Pharma Pulse, new trials confirm AI chatbots are a successful and sustainable tool for increasing pneumococcal vaccine uptake among older adults and much more.

The entrepreneur urges the Trump administration to eliminate costly FDA generic drug fees, part of a broader push to scale domestic production, address shortages, and challenge PBM-driven pricing models.

In today’s Pharma Pulse, a structured exercise program is proven to manage persistent long COVID symptoms, while Regeneron commits $150 million upfront to Tessera for a gene writing therapy targeting AATD, and Strive Pharmacy invests in a massive Arizona facility to boost national personalized medicine capacity.

The agreement includes exemptions for UK-produced drugs and medical devices from Section 232 tariffs, but mandates a significant change to the UK's NICE value appraisal framework.

The Trump administration has announced new negotiated Medicare prices for a second wave of blockbuster treatments, yielding an estimated $12 billion in savings for 2027, when compared to Medicare’s 2024 net spending.

As the federal government prepares to release newly negotiated Medicare drug prices, weight-loss blockbusters Ozempic and Wegovy remain in the spotlight, while CMS advances a model to cut Medicaid spending and broaden access to affordable treatments.

Moderna secures a $1.5 billion loan to enhance financial flexibility while targeting a 10% revenue growth by 2026, focusing on innovative mRNA therapies.

Abbott's deal shakes up cancer diagnostics, new research confirms pandemic fatigue's impact on public compliance, and more in today’s Pharma Pulse.

While studies reveal the complex, varying, and long-lasting burden of Long COVID symptoms, biopharma giants Novartis and Moderna commit significant capital to new facilities, reinforcing US drug supply resilience in today’s Pharma Pulse.

As part of its multi-billion-dollar investment, the company will be creating a unified, end-to-end manufacturing campus spanning biologics, gene therapy, solid dosage, and packaging operations.

A US-China Economic and Security Review Commission report highlights China’s tightening grip on APIs, biomanufacturing, and R&D services, raising alarms about supply chain vulnerabilities, data transparency gaps, and the urgent need for US policy action.
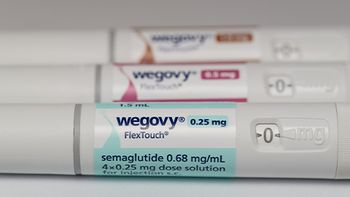
The company is launching new self-pay pricing options, expanded savings programs, and partnerships with telehealth and retail providers. all while its obesity portfolio gains momentum under the FDA’s National Priority Voucher program.

This episode of Pharma Pulse discusses how new analysis shows poor treatment and monitoring rates among young adults with high cholesterol, while national efforts ramp up to identify pharmacy deserts—and one US pharmacy becomes the first to accept cryptocurrency for prescriptions.













