
Data & Technology
Latest News


Addressing continued hurdles in patient adherence

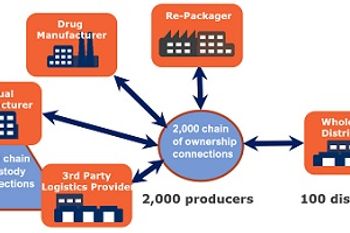
Steady progress is occurring in the global effort to track pharmaceutical distribution, even as new technical and business opportunities appear

PDG will hold a two-day webinar showcasing participating organizations

Robert Wood Johnson Foundation-sponsored study assesses the situation

Extension of the company’s Pathways program seeks to offer a richer and deeper patient interaction

Industry consortium is poised to manage pharma supply-chain interoperability governance

Kit Check data show value of track-and-trace technology, especially during the Covid-19 pandemic

Revenue leakage in contracting and sales execution affects 95% of respondents

Acquisition of Cumberland Life Sciences boosts its client base and range of services

For sessions with co-workers or clients, consider these recommendations

Level 1-3 serialization with a cloud-based system is up and running in a day

ICER will expand use of RWE, as the Federal ONC calls for broader use of healthcare data

Now two-plus years on, the dispute over physician databases shows no sign of resolution

TruTrace tackles the marijuana supply chain; potential for pharma traceability

Transforming the container to an interactive portal

Global Track & Trace Forum commemorates its 10th anniversary

Version 5.0 of Resilix platform includes more use-cases

Updates its Patient Connect platform with new interactive capabilities

Merck KGaA will use aspects of it to more closely manage its supply chain


$430-million all-cash acquisition signals Veeva’s move into consumer tracking

Other adoptions are in the works, according to Vantage Solutions

LedgerDomain’s 'KitChain' uses iOS to interface with blockchain network

